预约演示
更新于:2025-09-28
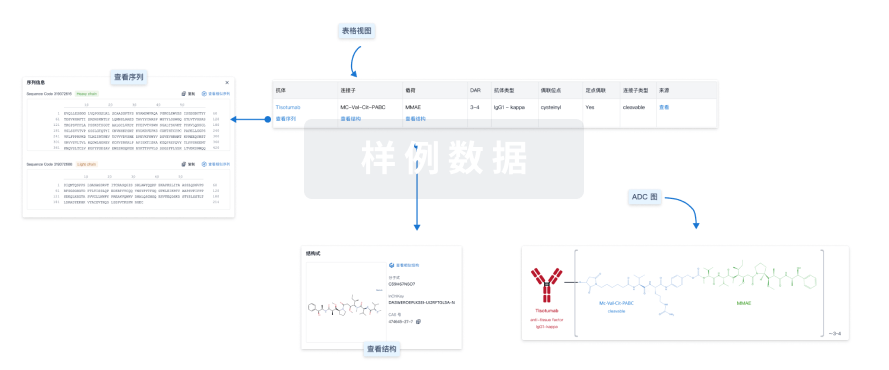
Brentuximab Vedotin
维布妥昔单抗
更新于:2025-09-28
概要
基本信息
药物类型 ADC |
别名 Brentuximab、Brentuximab Vedotin (Genetical Recombinatin)、Brentuximab vedotin (genetical recombination) (JAN) + [18] |
作用方式 抑制剂 |
作用机制 CD30抑制剂(肿瘤坏死因子受体超家族成员8抑制剂)、微管蛋白抑制剂 |
原研机构 |
最高研发阶段批准上市 |
最高研发阶段(中国)批准上市 |
特殊审评突破性疗法 (美国)、加速批准 (美国)、孤儿药 (美国)、孤儿药 (欧盟)、优先审评 (中国)、孤儿药 (韩国) |
登录后查看时间轴
结构/序列
使用我们的ADC技术数据为新药研发加速。
登录
或
Sequence Code 9923046L

来源: *****
Sequence Code 13006940H

来源: *****
关联
205
项与 维布妥昔单抗 相关的临床试验NCT07178457
Brentuximab Vedotin in CutAneous T-cell Lymphomas (CTCL): Post-allogeneic Hematopoietic Stem Cell Transplant Maintenance
Allogeneic hematopoietic stem cell transplantation (alloHSCT) showed efficacy in advanced-stage, high-risk, treatment sensitive cutaneous T-cell lymphomas (CTCL) in a prospective, propensity score-matched controlled study (CUTALLO) published by our group in the Lancet in 2023. Nevertheless, it is associated with a high rate of early relapse, with 2-year progression-free survival around 30%. Brentuximab vedotin (BV) has shown efficacy in the treatment of CD30-expressing CTCL after at least one prior systemic treatment in the ALCANZA trial and is market approved in this indication in Europe and the United States of America. BV has been successfully used as salvage treatment in the post-transplant setting in advanced CTCL. It has been shown that 90% of CTCL express CD30. To reduce the incidence of post-allograft relapse, we propose to assess the routine use of BV post-allograft in adult patients with advanced CD30-expressing mycosis fungoides-CTCL, who have received at least one line of prior systemic therapy, compared to placebo. Switch will be allowed from placebo to BV in case of disease progression. This project is supported by well-organized research networks with a strong track-record of published results in the field: French Study Group on Cutaneous Lymphomas (GFELC, Groupe Français d'Etude des Lymphomes Cutanés), INCa-labelled national rare cancers network; and the French Society of Bone Marrow Transplantation and CellTherapy. In the post-transplant setting, the current state-of-the-art practice is to treat patients once they have relapsed post-allogeneic transplant, whereas no prophylactic treatment is given at the time in the absence of characterized disease relapse. This ethically and scientifically justifies the proposal to evaluate whether earlier, prophylactic treatment with BV increases progression-free survival compared to placebo.
开始日期2025-11-01 |
NCT07145125
Real-life Efficacy and Toxicity of Brentuximab-Vedotin Associated With Bendamustine in Patients With Relapsed or Refractory Hodgkin Lymphoma
Approximately 20% of patients diagnosed with Hodgkin lymphoma will eventually experience progression or relapse after first-line treatment, which carries a significant risk of disease-related death. Although several pilot studies have demonstrated high rates of sustained response with the combination of brentuximab vedotin (BV) and chemotherapy, consistent real-world data are still lacking. Moreover, there is no universally accepted salvage chemotherapy regimen in this setting, and clinical practices vary across centers. This study aims to describe the efficacy and toxicity of the BV + bendamustine (B2) regimen in patients with relapsed/refractory Hodgkin lymphoma (R/R HL) treated in France over a 10-year period, with or without an attempt at consolidative transplantation.
开始日期2025-09-01 |
申办/合作机构- |
NCT07002216
A Phase 2 Trial of Abbreviated Brentuximab Vedotin, Etoposide, Cyclophosphamide, Adriamycin, Dacarbazine, and Dexamethasone (BrECADD) Therapy in Stage 2 B-IV Hodgkin Lymphoma
The purpose of this study is to further assess the efficacy and tolerability of a regimen of Brentuximab Vedotin, Etoposide, Cyclophosphamide, Doxorubicin, Dacarbazine, and Dexamethasone (BrECADD) in patients with Stage 2 B-IV Hodgkin Lymphoma (HL) with an exploratory objective to assess the clinical utility of Circulating tumor DNA (ctDNA) as a biomarker for minimal residual disease (MRD) and depth of treatment response.
开始日期2025-07-15 |
申办/合作机构  University of Miami University of Miami [+1] |
100 项与 维布妥昔单抗 相关的临床结果
登录后查看更多信息
100 项与 维布妥昔单抗 相关的转化医学
登录后查看更多信息
100 项与 维布妥昔单抗 相关的专利(医药)
登录后查看更多信息
1,536
项与 维布妥昔单抗 相关的文献(医药)2025-12-01·Blood Research
Treatment of older patients with Hodgkin lymphoma
Review
作者: Park, Chung Hyun ; Cho, Hyunsoo ; Kim, Soo-Jeong
Abstract:
Older patients with classic Hodgkin lymphoma (HL) often experience poor outcomes due to age-related comorbidities and treatment-related toxicity. Comprehensive geriatric assessment and supportive care measures, including pre-phase corticosteroids, growth factor prophylaxis, and organ function monitoring, are essential for optimizing treatment tolerance in this vulnerable patient population. Recent phase III trial (S1826) demonstrated that nivolumab plus doxorubicin, vinblastine, and dacarbazine (Nivo + AVD) significantly improves progression-free survival and is better tolerated than brentuximab vedotin (BV) + AVD, particularly in patients over 60 years of age. Given its efficacy and reduced toxicity, Nivo + AVD is likely to become a key treatment option for fit older patients with HL. For frail patients, chemo-free approaches with BV and checkpoint inhibitors remain viable alternatives. Future research should refine fitness-based treatment strategies, integrate novel agents, and enhance supportive care to improve outcomes and minimize treatment-related toxicity in this population.Graphical Abstract
2025-10-14·Blood Advances
Advances and updates in pediatric anaplastic large cell lymphoma
Review
作者: Lowe, Eric ; Kamdar, Kala ; Marks, Lianna J.
Abstract:
Anaplastic large cell lymphoma (ALCL) is a rare form of mature T-cell non-Hodgkin lymphoma. In pediatric patients, most cases are anaplastic lymphoma kinase (ALK) positive. Despite intensive multiagent chemotherapy regimens, treatment failure rates remain at 25% to 30%. Recent advancements in targeted therapies, notably ALK inhibitors and the anti-CD30 antibody-drug conjugate brentuximab vedotin have demonstrated substantial activity in relapsed and refractory settings. Molecular detection of minimal disseminated disease (MDD) and minimal residual disease (MRD) offers improved prognostic stratification. For patients with relapsed or refractory disease, targeted therapies have increased treatment options, but more work needs to be done to define optimal treatment regimens, duration, and need for hematopoietic stem cell transplantation in this group. Immune therapies such as checkpoint inhibitors or chimeric antigen receptor T-cell therapy provide additional therapeutic options. Incorporating targeted therapies and MDD/MRD assessments into clinical trials could significantly improve outcomes for pediatric and adolescent patients with ALCL.
2025-09-20·JOURNAL OF CLINICAL ONCOLOGY
Positron Emission Tomography–Guided Brentuximab Vedotin, Etoposide, Cyclophosphamide, Doxorubicin, Dacarbazine, and Dexamethasone in Older Patients With Advanced-Stage Classic Hodgkin Lymphoma: A Prospective, Multicenter, Single-Arm, Phase II Cohort of the German Hodgkin Study Group HD21 Trial
Article
作者: Ferdinandus, Justin ; Balabanov, Stefan ; Mey, Ulrich ; Hüttmann, Andreas ; Hertzberg, Mark ; Roncolato, Fernando ; Tharmaseelan, Hishan ; Ko, Yon-Dschun ; Eichenauer, Dennis A. ; Wille, Kai ; Trenker, Corinna ; Staib, Peter ; Kerkhoff, Andrea ; Borchmann, Peter ; Kobe, Carsten ; Fosså, Alexander ; Dresel, Irmgard ; Keil, Felix ; Kreissl, Stefanie ; Kaul, Helen ; Armytage, Tasman ; Klapper, Wolfram ; Eich, Hans-Theodor ; Moosmann, Peter ; Schneider, Gundolf ; Hitz, Felicitas ; Baues, Christian ; Jablonski, Janina ; von Tresckow, Bastian ; Fuchs, Michael ; Hellmuth, Johannes C. ; Bröckelmann, Paul J. ; Hahn, Dennis ; Schwarz, Michaela ; Hertenstein, Bernd
PURPOSE:
Positron emission tomography (PET)-guided therapy with 4-6 cycles of brentuximab vedotin, etoposide, cyclophosphamide, doxorubicin, dacarbazine, and dexamethasone (BrECADD) is highly effective in younger patients with advanced-stage classic Hodgkin lymphoma (AS-cHL). We report feasibility and efficacy of PET-guided BrECADD as first-line treatment in older patients with AS-cHL.
PATIENTS AND METHODS:
Patients with AS-cHL aged 61-75 years were enrolled in a phase II single-arm cohort of the HD21 trial (ClinicalTrials.gov identifier:
NCT02661503
). Patients with negative PET/computed tomography after 2×BrECADD (PET2) received a total of 4×BrECADD, while PET2-positive patients received 6×BrECADD. The primary end point was the centrally reviewed complete remission (CR) rate after the end of chemotherapy (EOC). Secondary end points included feasibility, adverse events, treatment-related morbidity (TRMB), progression-free survival (PFS), overall survival (OS), and health-related quality of life (HRQoL).
RESULTS:
Between June 2020 and April 2023, 85 patients were enrolled, of whom 83 with a median age of 67 years (range, 61-75) were analyzed in the intention-to-treat cohort. Most prevalent ≥grade 3 toxicities included leukopenia (n = 80 [96%]), thrombocytopenia (n = 71 [86%]), anemia (n = 57 [69%]), and febrile neutropenia (n = 46 [55%]). Forty-eight (60%) of 80 patients with centrally reviewed PET2 were scheduled for 4×BrECADD and 32 (40%) for 6×BrECADD. Of these, 71 patients (89%) received the target number of cycles. Sixty-eight patients (82%; 95% CI, 72 to 90) achieved CR at EOC. PFS and OS estimates at 2 years were 91.5% (95% CI, 85 to 98) and 90.8% (95% CI, 84 to 98), respectively. No death was attributed to study treatment. Initially, impaired HRQoL scores improved during follow up and on average reached population reference values.
CONCLUSION:
PET-guided BrECADD in older patients is feasible and effective. With a PFS rate on par with that of younger patients, short duration, and limited anthracycline exposure, BrECADD is a valuable treatment option also for older patients with AS-cHL.
618
项与 维布妥昔单抗 相关的新闻(医药)2025-09-23
·抗体圈
摘要:抗体药物偶联物(ADC)作为癌症治疗领域的 “生物导弹”,凭借单克隆抗体的精准靶向性与细胞毒性载荷的强效抗肿瘤作用,已成为发展最快的靶向治疗药物之一。截至 2024 年 6 月,全球已有 15 款 ADC 药物获批用于实体瘤与血液系统肿瘤治疗,另有超 100 项不同阶段临床试验正在推进。然而,耐药性的出现严重制约了 ADC 的临床疗效。本文将从 ADC 的结构设计、作用机制与世代发展入手,系统梳理抗原 - 抗体结合异常、药物运输障碍、溶酶体功能异常等六大类耐药机制,并介绍新型 ADC 研发、联合治疗等应对策略,同时总结潜在的耐药预测生物标志物,为 ADC 药物的临床应用与未来发展提供参考。
一、ADC 药物:癌症治疗的 “精准导弹”1.1 ADC 的结构组成:三大核心部件
ADC 药物由单克隆抗体(mAb)、连接子(Linker) 和细胞毒性载荷(Payload) 三部分通过共价键连接而成,各部件功能协同,共同决定药物的疗效与安全性(图 1)。
单克隆抗体:负责 “导航”,通过识别肿瘤细胞表面特异性抗原(如 HER2、CD33),将药物精准递送至肿瘤部位,同时还能通过抗体依赖的细胞介导的细胞毒性作用(ADCC)、补体依赖的细胞毒性作用(CDC)等直接发挥抗肿瘤活性。早期 ADC 使用鼠源抗体易引发免疫反应,目前临床多采用全人源或人源化 IgG1 亚型抗体,兼具高亲和力与低免疫原性。
连接子:作为 “桥梁”,需在血液循环中保持稳定以避免载荷过早释放损伤正常组织,到达肿瘤细胞后则需高效断裂释放载荷。根据载荷释放机制,连接子可分为可切割型(利用肿瘤微环境与血液的差异,如酸性 pH、特定蛋白酶切割)与不可切割型(需进入溶酶体后通过蛋白水解反应释放载荷),其亲水性还会影响 ADC 的聚集性与肝毒性。
细胞毒性载荷:是 “弹头”,需具备高细胞毒性(亚纳米浓度即可杀伤肿瘤细胞)、低分子质量(减少免疫原性)与可修饰性(便于与连接子结合)。目前临床常用载荷包括微管抑制剂(如 MMAE、DM1)、DNA 作用抑制剂(如 PBD 二聚体)与拓扑异构酶 I 抑制剂(如 DXd、SN38)三类。
此外,药物抗体比率(DAR) 是评估 ADC 质量的关键指标,代表单个抗体偶联的载荷数量。DAR 过低会导致抗肿瘤活性不足,过高则可能增加毒性与循环清除速度,当前主流 ADC 的 DAR 多控制在 2-8 之间。1.2 ADC 的作用机制:多途径协同抗肿瘤
ADC 进入体内后,通过静脉注射快速分布,凭借抗体的长半衰期到达肿瘤部位,其抗肿瘤作用主要通过四大途径实现(图 2):
靶向药物递送:抗体与肿瘤抗原结合后,形成的 ADC - 抗原复合物通过网格蛋白介导的内吞作用进入细胞,随后经内体转运至溶酶体,连接子断裂释放载荷,载荷作用于 DNA 或微管,诱导肿瘤细胞凋亡。
抗体固有活性:抗体的 Fc 段可与效应细胞表面的 Fc 受体结合,介导 ADCC、CDC 与抗体依赖的吞噬作用(ADCP),直接杀伤肿瘤细胞。例如,用于 HER2 阳性乳腺癌的 ADC 药物 T-DM1,其抗体部分可抑制 HER2 信号通路,同时触发 ADCC。
旁观者效应:若释放的载荷具有细胞膜渗透性(如 DXd、MMAE),可扩散至周围未表达靶抗原的肿瘤细胞,扩大杀伤范围,尤其对肿瘤异质性导致的耐药具有重要作用。获批药物 DS-8201(HER2 阳性癌症)与 Sacituzumab Govitecan(三阴性乳腺癌)均能有效诱导旁观者效应。
肿瘤微环境调节:部分 ADC 可通过改变肿瘤微环境(如招募免疫细胞)增强抗肿瘤免疫,为联合免疫治疗奠定基础。1.3 ADC 的世代发展:从雏形到成熟
经过数十年迭代,ADC 已发展至第三代,各世代在抗体、连接子、载荷与偶联技术上逐步优化(图 3):
第一代 ADC(2000 年获批的 Gemtuzumab ozogamicin 为代表):采用鼠源 / 嵌合抗体,连接子稳定性差,载荷毒性较低,且载荷与抗体随机偶联导致 DAR 异质性高(1-8),存在半衰期短、免疫原性强、治疗窗口窄等问题。
第二代 ADC(如 Brentuximab vedotin、T-DM1):改用人源化 IgG1 抗体,优化连接子稳定性(可切割或不可切割型),选用更强效的载荷(如 MMAE、DM1),DAR 分布更均匀(4-8),但仍存在耐受性低、高 DAR 药物清除快、脱靶毒性等问题。
第三代 ADC(如 DS-8201、Polatuzumab vedotin):采用全人源抗体或抗原结合片段,连接子增加亲水性基团以减少聚集,载荷毒性更强(如 DXd、PBD 二聚体),DAR 控制在 2-4 之间,同时提升了稳定性与药代动力学性能,在低抗原表达肿瘤中也能发挥作用。
截至 2024 年 6 月,全球获批的 15 款 ADC 药物中,约半数用于实体瘤(如乳腺癌、胃癌),半数用于血液系统肿瘤(如白血病、淋巴瘤),其核心信息如表 1 所示;另有超 100 款 ADC 处于临床研发阶段,靶点涵盖 cMET、Trop-2、CLDN18.2 等,部分药物已进入 II/III 期临床试验(表 2)。
表 1 已获批 ADC 药物汇总
表格来源:原文 Table 1,注:HER2 = 人表皮生长因子受体 2;Trop-2 = 滋养层细胞表面抗原 2;MMAE = 单甲基澳瑞他汀 E;DXd = 依沙替康衍生物;SN38 = 伊立替康活性代谢物
表 2 处于不同临床试验阶段的 ADC 药物(部分)
表格来源:原文 Table 2,注:cMET = 间质上皮转化因子;CEACAM5 = 癌胚抗原相关细胞黏附分子 5;CLDN18.2 = 紧密连接蛋白 18.2;EGFR = 表皮生长因子受体;vc = 缬氨酸 - 瓜氨酸肽;DXd = 依沙替康衍生物;MMAE = 单甲基澳瑞他汀 E二、ADC 耐药的六大核心机制
尽管 ADC 疗效显著,但多数患者在治疗后会逐渐出现耐药,其机制复杂且涉及 ADC 作用的全流程,主要可分为六大类(图 4)。
2.1 抗原 - 抗体结合异常:ADC “导航失灵”
抗原 - 抗体结合是 ADC 发挥作用的第一步,该过程异常会直接导致药物无法精准靶向肿瘤细胞,主要包括四种情况:
靶抗原表达降低或丢失:长期靶向治疗可能导致肿瘤细胞下调靶抗原表达。例如,HER2 阳性乳腺癌患者经多线抗 HER2 治疗后,HER2 表达降低,导致 ADC 药物 T-DM1 无法结合并内化,载荷释放减少;霍奇金淋巴瘤中,耐药细胞系的 CD30 表达量显著低于敏感细胞系,使 CD30 靶向 ADC(Brentuximab vedotin)失效。
外周靶抗原消耗 ADC:血液或肿瘤微环境中的游离抗原会与 ADC 结合,消耗药物量,降低肿瘤部位的药物浓度。例如,白血病患者外周血中高表达 CD33 的白血病细胞会结合 ADC 药物 Gemtuzumab ozogamicin,导致骨髓中药物浓度不足;B 细胞淋巴瘤细胞分泌的含 CD20 的外泌体,会 “捕获” 抗 CD20 ADC,使其无法作用于肿瘤细胞。
抗体结合位点受损:肿瘤细胞可能通过抗原截断、突变或掩盖结合表位,阻碍抗体结合。例如,截断型 HER2(p95HER2)缺失曲妥珠单抗结合的胞外域,但仍能促进肿瘤进展,导致 HER2 靶向 ADC 耐药;肿瘤细胞分泌的 MUC4 蛋白可掩盖 HER2 上的抗体结合位点,降低 T-DM1 的结合效率。
肿瘤异质性:同一肿瘤中不同细胞的抗原表达存在差异,部分低 / 无抗原表达的细胞可逃避 ADC 杀伤。例如,HER2 阳性乳腺癌中,若 5%-50% 肿瘤细胞为 HER2 阴性,患者对 T-DM1 的病理完全缓解率显著降低,复发风险升高。2.2 药物运输障碍:ADC “无法入胞”
ADC 与抗原结合后,需通过内吞作用进入细胞并转运至溶酶体,此过程异常会导致药物无法有效释放载荷:
内吞效率降低与回收增加:内吞相关蛋白(如 Endophilin A2)下调会减少 ADC - 抗原复合物的内吞。例如,HER2 阳性乳腺癌细胞中,Endophilin A2 沉默会抑制 HER2 内吞,降低 T-DM1 的细胞毒性;部分抗原(如 HER2)内吞后更易被回收至细胞膜,而非转运至溶酶体,导致载荷无法释放。
内吞途径改变:细胞内吞主要分为网格蛋白介导与非网格蛋白介导(如小窝蛋白介导)。敏感细胞中,ADC 多通过网格蛋白介导的内吞进入溶酶体;而耐药细胞中,ADC 可能通过小窝蛋白 - 1(CAV1)介导的内吞进入小窝体(中性 pH 环境),无法触发连接子断裂与载荷释放,导致耐药。2.3 溶酶体功能异常:ADC “弹头无法释放”
不可切割型 ADC 需依赖溶酶体的酸性环境与蛋白酶水解释放载荷,溶酶体功能异常会直接影响载荷释放:
溶酶体 pH 升高:溶酶体酸性环境由空泡型 H+-ATP 酶(V-ATPase) 维持,其活性降低会导致溶酶体 pH 升高,抑制不可切割型 ADC 的水解。例如,T-DM1 耐药细胞中 V-ATPase 活性下降,溶酶体 pH 升高,T-DM1 代谢受阻;而可切割型 ADC(如曲妥珠单抗 - vc-MMAE)因连接子可在胞内早期阶段断裂,受此影响较小。
溶酶体隔离载荷:部分载荷(如疏水性弱碱药物)进入溶酶体后会因酸性环境带电,被隔离在溶酶体内无法进入细胞质发挥作用。例如,SN38(Sacituzumab Govitecan 的载荷)在耐药细胞中易被溶酶体隔离;溶酶体膜蛋白 SLC46A3 可促进载荷(如 DM1)从溶酶体转运至细胞质,其下调会导致非可切割型 ADC 耐药。2.4 载荷相关耐药:ADC “弹头失效”
ADC 的载荷本质是化疗药物,其耐药机制与传统化疗类似:
载荷靶点改变:载荷作用靶点的突变或表达变化会降低药物敏感性。例如,微管抑制剂(MMAE、DM1)的靶点是微管蛋白,耐药细胞中 β- 微管蛋白亚型(βII、βIII)表达升高,或微管蛋白乙酰化水平降低,导致药物无法结合;拓扑异构酶 I 抑制剂(DXd、SN38)的靶点是拓扑异构酶 I,其基因突变或核定位改变会减少药物诱导的 DNA 损伤,导致耐药。
ABC 转运体介导的药物外排:ATP 结合盒(ABC)转运体(如 MDR1)可将胞内载荷泵出细胞,降低药物浓度。例如,Gemtuzumab ozogamicin 耐药细胞中 MDR1 表达升高,导致刺孢霉素外排增加;T-DM1 耐药细胞中多种 ABC 转运体上调,减少 DM1 在胞内的积累。2.5 肿瘤细胞存活增强:ADC “无法诱导凋亡”
肿瘤细胞通过调控细胞周期与凋亡通路,增强自身存活能力,抵抗 ADC 杀伤:
细胞周期动力学改变:微管抑制剂类 ADC(如 T-DM1)通过抑制微管聚合,使细胞周期停滞于纺锤体组装 checkpoint(SAC),诱导凋亡。耐药细胞中, Polo 样激酶 1(PLK1)过表达或周期蛋白 B1 降解,可绕过 SAC checkpoint,导致细胞周期继续推进(即 “有丝分裂滑移”),逃避凋亡。
凋亡通路激活受阻:抗凋亡蛋白(如 Bcl-2、Bcl-xL)过表达或促凋亡蛋白(如 Bax、Bak)下调,会抑制 ADC 诱导的凋亡。例如,非霍奇金淋巴瘤细胞中,Bcl-xL 过表达会导致 CD79b 靶向 ADC(Polatuzumab vedotin)耐药;Bcl-2/xL 抑制剂(如 ABT-263)可恢复 ADC 的细胞毒性。2.6 肿瘤微环境调控:ADC “发挥环境恶劣”
肿瘤微环境中的细胞因子、生长因子与免疫抑制细胞,可通过多种途径促进 ADC 耐药:
RTK 信号通路冗余:肿瘤细胞或基质细胞分泌的生长因子(如 NRG-1β、HGF)可激活替代受体酪氨酸激酶(RTK)通路(如 PI3K/Akt),补偿 ADC 对靶 RTK(如 HER2)的抑制。例如,NRG-1β 可促进 HER3 与 HER2 异二聚化,激活 PI3K 通路,降低 T-DM1 的疗效;HGF 可激活 c-Met 通路,导致 EGFR 靶向 ADC 耐药。
免疫抑制微环境:肿瘤微环境中的调节性 T 细胞(Treg)、髓系抑制细胞(MDSC)会抑制免疫反应,削弱 ADC 的 ADCC 与 CDC 效应。例如,CD8+T 细胞耗竭会显著降低 ADC 疗效;而 ADC 与免疫检查点抑制剂(如 PD-1 抑制剂)联合,可招募 CD8+T 细胞,增强抗肿瘤免疫。三、突破 ADC 耐药:从药物研发到临床策略
针对 ADC 耐药的多机制,目前主要通过新型 ADC 研发与联合治疗两大方向突破,同时探索生物标志物指导精准用药。3.1 新型 ADC 研发:优化 “导弹” 性能
选用不易外排的载荷:开发 ABC 转运体非底物的载荷,避免药物外排导致的耐药。例如,DS-8201 采用拓扑异构酶 I 抑制剂 DXd,不易被 MDR1 外排,可克服 T-DM1 在 HER2 阳性胃癌中的耐药;SGN-CD33A 采用 PBD 二聚体作为载荷,在耐药 AML 模型中仍能发挥活性。
增强旁观者效应:设计具有细胞膜渗透性的中性载荷(如 DM4),或优化连接子亲水性,扩大 ADC 对异质性肿瘤的杀伤范围。例如,IMGN853(FRα 靶向 ADC)采用亲水性连接子,旁观者效应增强,对 FRα 低表达肿瘤仍有效。
双靶点/双载荷 ADC:双靶点 ADC(如靶向 HER2 与 CD63)可同时结合两种抗原,减少因单一抗原丢失导致的耐药;双载荷 ADC(如同时携带微管抑制剂与 DNA 损伤剂)可通过两种机制杀伤肿瘤,降低耐药风险。例如,新型 HER2 靶向双载荷 ADC 在 HER2 异质性乳腺癌模型中,疗效显著优于单载荷 ADC。
抗体小型化:传统 IgG 抗体(150 kDa)难以穿透实体瘤,通过去除 Fc 段获得的小型抗体(如单域抗体),可增强肿瘤穿透性,但需通过 PEG 修饰延长体内半衰期。例如,人源单域抗体偶联 ADC 在实体瘤模型中,肿瘤穿透率与杀伤活性均优于传统 ADC。3.2 联合治疗:多机制协同抗肿瘤
ADC与靶向药物联合:针对 RTK 信号冗余或凋亡通路激活,联合 ADC 与靶向药物可增强疗效。例如,T-DM1 联合拉帕替尼(HER2 抑制剂)可克服 HER2 耐药,提高 HER2 阳性乳腺癌的客观缓解率;ADC 联合 Bcl-2/xL 抑制剂(如 ABT-263)可恢复耐药细胞的凋亡敏感性。
ADC 与免疫检查点抑制剂联合:ADC 可诱导免疫原性细胞死亡,招募免疫细胞,与 PD-1/PD-L1、CTLA-4 抑制剂联合可协同增强免疫应答。例如,DS-8201 联合 PD-1 抑制剂在 HER2 阳性非小细胞肺癌中,客观缓解率显著高于单药;多项 I/II 期临床试验(如 NCT05547321、NCT05701527)正在探索此类联合方案的疗效。
ADC 与化疗联合:针对肿瘤异质性,联合 ADC 与化疗可覆盖低 / 无抗原表达的肿瘤细胞。例如,Sacituzumab Govitecan 联合铂类化疗在三阴性乳腺癌中,无进展生存期显著延长;但需注意化疗可能增加毒性,需平衡疗效与安全性。3.3 耐药预测生物标志物:实现精准用药
目前临床主要通过检测靶抗原表达预测 ADC 疗效,同时探索更多潜在生物标志物:
靶抗原表达:HER2、Trop-2、FRα 等抗原的表达水平是 ADC 用药的基础指标。例如,c-Met 表达水平可预测 c-Met 靶向 ADC(SHR-A1403)的疗效;Trop-2 高表达患者对 Sacituzumab Govitecan 的响应率更高。
内吞与溶酶体相关蛋白:RAB5A(调控内体融合)高表达的 HER2 阳性乳腺癌患者,对 T-DM1 的预后更好;SLC46A3(溶酶体载荷转运蛋白)下调提示非可切割型 ADC 耐药。
凋亡与信号通路相关蛋白:Bcl-xL 过表达提示 ADC 耐药;V-ATPase 活性降低可预测不可切割型 ADC(如 T-DM1)耐药。
DNA 修复相关蛋白:同源重组修复功能正常的患者,对 SN38 类 ADC(如 Sacituzumab Govitecan)的耐药风险更高;SLFN11(DNA 复制 checkpoint 蛋白)下调提示 PBD 二聚体类 ADC 耐药。四、总结与展望
ADC 药物凭借精准靶向与强效杀伤的优势,已成为癌症治疗的重要手段,15 款获批药物与超 100 项临床试验彰显其快速发展态势。然而,多机制耐药仍是制约 ADC 临床疗效的核心挑战,涉及抗原结合、药物运输、溶酶体功能、载荷作用、细胞存活与肿瘤微环境六大环节。
未来,ADC 的发展需聚焦三大方向:一是通过优化抗体、连接子与载荷,开发具有更强靶向性、更稳定连接与更广杀伤范围的新型 ADC;二是基于耐药机制,设计 ADC 与靶向药物、免疫治疗、化疗的联合方案,实现多机制协同;三是探索更多精准的耐药预测生物标志物,通过动态监测(如液体活检)指导个体化治疗。
随着对 ADC 耐药机制的深入理解与技术的持续创新,相信这一 “生物导弹” 将在癌症治疗中发挥更大作用,为患者带来更多生存获益。
抗体药物偶联物
2025-09-21
·抗体圈
摘要:抗体偶联药物(ADC)是由单克隆抗体和有效载荷通过连接子偶联而成的新型抗体药物。ADC 药物能高选择性地杀灭肿瘤细胞,并有效降低小分子载荷对正常组织或器官的毒性作用。本文从 ADC 药物的发展历史、主要组成、热门靶点、获批情况及质量控制等方面进行综述,以期为后续的 ADC 研发提供初步参考。
抗体偶联药物(antibody-drug conjugates, ADC)是由对肿瘤细胞表面特异性抗原有靶向作用的抗体或抗体片段与有效载荷(payload)通过连接子(linker)偶联而成的一类新型抗体药物。抗体分子发挥靶向投递作用(抗体介导或内吞等),有效载荷发挥杀伤肿瘤细胞效应(DNA 插入或抑制微管合成等)。ADC 兼具传统小分子药物的强效作用和抗体药物的靶向性,能高选择性地杀灭肿瘤细胞,同时可降低小分子载荷对正常组织或器官的毒性作用。ADC 是当前全球研发的热点,其基本结构包含抗原、抗体、连接子和细胞毒载荷四个部分。1 ADC 的发展历史
上世纪初,德国科学家 Paul Ehrlich(诺贝尔生理学 / 医学奖获得者)首次提出了 “魔术子弹” 假说,认为可以将药物靶向递送至病原体,灭杀病原体而不伤害正常细胞。1958 年,MATHE 等首次将抗鼠免疫球蛋白与甲氨蝶呤进行偶联用于白血病治疗,尽管未能最终开发成药,但拓展了使用偶联药物治疗疾病的可能性。1975 年,KOHLER 和 MILSTEIN 发明了单克隆抗体(monoclonal antibody, mAb),促进了 IgG 单抗 ADC 逐步进入临床验证阶段。
2000 年,首个 ADC 吉妥珠单抗(Mylotarg)获批上市,用于治疗急性髓系白血病(AML),因可导致严重的肝静脉闭塞症于 2010 年撤市。2011 年,首个治疗实体瘤(霍奇金淋巴瘤)的 ADC 维布妥昔单抗(Adcetris)获批上市。2013 年,首个治疗人表皮生长因子受体 2(Her2)阳性转移性乳腺癌的 ADC 恩美曲妥珠单抗(Kadcyla)获批上市。此后,ADC 迎来全球的研发热潮,获批上市药物逐年持续增加,填补了多个以往未能满足的临床需求。
从总体上看,ADC 的开发大致经历了三个阶段:
第一代 ADC:采用鼠源单抗(后逐渐过渡为人源化单抗),以抗肿瘤抗生素或甲氨蝶呤等为载荷,采用腙键 / 二硫键连接子,在抗体的赖氨酸残基处进行偶联,以吉妥珠单抗为代表。吉妥珠单抗使用奥唑米星(卡奇霉素衍生物)为细胞毒载荷,通过腙键 / 二硫键连接子与人源化 IgG4 单抗偶联。由于连接子在血液循环中易水解,脱靶毒性较高,该药物于 2010 年撤市(2017 年重新上市)。此阶段 ADC 在安全性和有效性方面存在较大缺陷,如免疫原性较高、药物 - 抗体比(drug-to-antibody ratio, DAR)分布宽、均一性较差、脱靶毒性较高等。
第二代 ADC:开始选择更高活性的细胞毒载荷、特异性更强的靶抗原,使用新的连接子及偶联方式等,以改善 ADC 的水溶性、血液系统中的稳定性及 DAR 分布的均一性,以恩美曲妥珠单抗为代表。恩美曲妥珠单抗使用微管抑制剂美登素衍生物(Mertansine)为细胞毒载荷,采用靶向 Her-2 的人源化曲妥珠单抗(Trastuzumab),以较稳定的连接子与载荷进行偶联。第二代 ADC 的疗效和安全性较第一代 ADC 有显著改善,但仍存在治疗窗狭窄、ADC 易聚集、DAR 分布宽且均一性差等缺陷。
第三代 ADC:逐步采用全人源化单抗、中等毒性的毒载荷,并继续拓展连接子和采用定点偶联等新技术,以改善 DAR 分布均一性和降低脱靶毒性作用,以德曲妥珠单抗(Enhertu)为代表。该阶段 ADC 的疗效和安全性等进一步改善。2 ADC 的组成
ADC 由具有靶向作用的抗体、有效载荷和连接子 3 个部分组成。理想的 ADC 应在血液循环中保持稳定,并能在靶部位精准释放出有效载荷,发挥其对肿瘤等靶细胞的杀伤作用。2.1 抗体
抗体是 ADC 发挥其效用的前提,ADC 所用的抗体经历了从鼠源性抗体→嵌合人源化抗体→全人源化抗体的历程。通常,ADC 所用抗体的大小及亲和力决定其穿透能力及内化效率,选择抗体时应关注高亲和力、低免疫原性、高稳定性和长半衰期等特性。
IgG 抗体是血清中最多的免疫球蛋白,由浆细胞产生,有 IgG1~IgG4 四种亚型,分子量约 150 ku,结构呈 “Y” 形(顶端的可变区为抗原结合位),是由两条重链和两条轻链组成的对称结构。轻链和重链两端分别为氨基端(N 端)和羧基端(C 端),重链分子量约 50 ku(由一个可变区(VH)、铰链区和三个恒定区 CH₁、CH₂、CH₃组成),轻链分子量约 25 ku(由一个可变区(VL)和一个恒定区(CL)组成)。重链间通过铰链区的二硫键连接,重链与轻链间通过重链 CH₁区与轻链区的链间二硫键连接。用合适的蛋白酶对抗体分子进行酶切,通常不会破坏其抗原结合位。
抗体分子上的赖氨酸、半胱氨酸、铰链区的巯基等,是抗体与连接子偶联的主要位点。IgG1 因在血液循环中的半衰期较长,可同时引发补体依赖的细胞毒性作用、抗体依赖的细胞毒性作用,以及抗体依赖性细胞介导的吞噬作用等,在 ADC 中的应用相对更广。2.2 连接子
ADC 中的连接子用于连接抗体与有效载荷,主要有可裂解连接子和不可裂解连接子两类。通常,连接子应在血液系统及非肿瘤部位保持稳定(避免药物产生脱靶毒性),并在肿瘤部位裂解释放出有效载荷,以发挥对靶细胞的杀伤作用。2.2.1 可裂解连接子
该类连接子主要利用肿瘤微环境与正常环境的差异来促成有效载荷的释放,包括以下几种类型:
pH 敏感型连接子:在偏酸性的肿瘤微环境下可水解,如腙基连接子、羧酸连接子等;
谷胱甘肽连接子:在血液循环中比较稳定,在肿瘤细胞内高谷胱甘肽浓度条件下易断裂为二硫键;
溶酶体酶敏感型连接子:在血液循环中较稳定,进入肿瘤细胞可被溶酶体酶裂解,如缬氨酸 - 瓜氨酸连接子、甘氨酸 - 甘氨酸 - 苯丙氨酸 - 甘氨酸连接子等;
β- 葡糖苷酸酶可裂解型连接子:此类连接子亲水性较强,可被溶酶体和肿瘤内的 β- 葡萄糖苷酸酶水解。2.2.2 不可裂解连接子
该类连接子对体内的常规化学或酶环境稳定,稳定性一般优于可裂解连接子。当 ADC 被内吞至肿瘤细胞后,在溶酶体内裂解释放出载荷 - 连接子 - 氨基酸残基,从而发挥对肿瘤细胞的杀伤作用,其全身毒性相对较低,治疗窗口相对较宽。例如硫醚连接子、琥珀酰亚胺基 - 4-(N - 马来酰亚胺基甲基)环己烷 - 1 - 甲酸酯连接子等。
2.3 有效载荷
ADC 的有效载荷包括具有细胞毒性的小分子,以及核素、多肽、寡义核苷酸等。本文参照国家药品监督管理局药品审评中心(CDE)发布的《抗体偶联药物药学研究与评价技术指导原则》(2024 年)等相关指导原则,重点对以细胞毒性小分子为有效载荷的 ADC 进行阐述。
细胞毒性的小分子一般为亲脂性物质,当 ADC 中结合的有效载荷过高时,可能导致 ADC 易聚集而被快速清除;结合的有效载荷过低时,可能无法达到所需疗效。因此,ADC 分子应有合理的 DAR。ADC 中目前常用的有效载荷主要为微管蛋白抑制剂及 DNA 抑制剂两大类。2.3.1 微管蛋白抑制剂
通过与肿瘤细胞中的微管蛋白结合来干扰微管的形成,从而杀伤肿瘤细胞。常用的微管抑制剂主要有:
美登素衍生物,如美坦辛(Mertansine)、拉维坦辛(Ravtansine)等;
澳瑞他汀衍生物,如单甲基奥瑞他汀 E(Monomethyl auristatin E)、单甲基奥瑞他汀 F(Monomethyl auristatin F)等。2.3.2 DNA 抑制剂
通过与肿瘤细胞的 DNA 双螺旋结合,使其 DNA 核酸链发生断裂、烷基化、插层或交联,进而导致肿瘤细胞死亡。常用的 DNA 抑制剂主要有:
抗肿瘤抗生素(如卡奇霉素、多柔比星);
DNA 拓扑异构酶抑制剂(如喜树碱衍生物伊立替康的活性代谢物 SN-38、吡咯并苯并二氮杂卓二聚体 PBD、烷化剂、甲氨蝶呤)等。2.4 偶联方式
偶联方式决定 ADC 的 DAR 及分布均一性,与 ADC 的生物活性及稳定性等密切相关。常用偶联方式有非定点偶联和定点偶联两大类。2.4.1 非定点偶联
通过抗体分子上的赖氨酸或半胱氨酸残基与连接子进行偶联。通常,赖氨酸偶联方式(与连接子上的琥珀酰亚胺酯等基团反应)形成的 ADC 的 DAR 分布较宽,均一性较差(单抗上赖氨酸残基≥20 个),异质性较强;半胱氨酸偶联(将抗体上链间二硫键还原成半胱氨酸残基,再与连接子上的马来酰亚胺等基团反应)形成的 ADC 的 DAR 分布均一性相对较好(单抗还原后的半胱氨酸残基≤8 个),异质性相对较低。2.4.2 定点偶联
该方式通过在抗体表面引入特殊活性基团,对抗体进行修饰后进行偶联,可减少 ADC 的异质性和提高治疗窗口,为目前第三代 ADC 最常用的偶联方式。目前常用的方式有以下几种:
在抗体的特定位置引入半胱氨酸残基,通过巯基进行偶联;
在抗体的特定位置引入与天然氨基酸结构类似的非天然氨基酸,利用其特定基团(如乙酰苯丙氨酸、对叠氮甲基 - L - 苯丙氨酸等)实现偶联;
在抗体的特定位置引入氨基酸序列,利用酶对相关氨基酸残基进行修饰后进行定点偶联;
对抗体相关位点的糖基(如 N297)进行修饰,获得可与连接子反应的基团(如唾液酸等),实现糖基定点偶联。3 ADC 热门靶点
靶点是 ADC 识别肿瘤细胞的关键,决定 ADC 的靶向特异性、适应证和内吞效率。在肿瘤细胞和正常细胞之间差异化表达的表面抗原(肿瘤部位高表达,正常组织中无表达或低表达),均可能是 ADC 的潜在靶点。目前已上市 ADC 靶点的简要信息如下表所示:
4 已上市 ADC 概况
目前,全球已有 16 个 ADC 获批上市,我国上市 9 个。其中,维迪西妥单抗(商品名:爱地希)和芦康沙妥珠单抗(商品名:佳泰莱)为我国自主研发和批准上市的 ADC。相关适应证包括血液瘤(如急性髓细胞白血病等)和实体瘤(如乳腺癌、非小细胞肺癌、尿路上皮癌等)。目前已上市 ADC 相关信息的简要概述如下表所示:
5 ADC 的质量控制
ADC 的组成结构复杂,其质量控制应结合组成及工艺等,从物理化学、免疫学、生物学等方面进行全面分析表征。按照 CDE 发布的《抗体偶联药物药学研究与评价技术指导原则》(2024)的要求,ADC 原液及制剂的质量控制应包括性状(外观、颜色等)、鉴别、DAR 值和药物载荷分布、纯度和杂质、生物学活性、含量及常规检查(无菌、不溶性微粒等)等项目。其中,DAR 及载荷分布、分子变异体及电荷异质体、相关杂质等均是影响 ADC 质量的关键指标。5.1 DAR 及载荷分布
DAR 是影响 ADC 疗效及安全性的关键质量属性,DAR 的分析包括 DAR 平均值测定和载荷分布测定。DAR 平均值反映每个单抗上连接的有效载荷的平均数量;载荷分布反映连接不同数目载荷的 ADC 分子相对于总 ADC 分子的占比,通常通过测定不同 DAR 值的抗体轻链和重链的相对含量来确定 ADC 的载荷分布情况。
DAR 常用的分析方法有:反相高效液相色谱法(RP-HPLC)、分子排阻色谱法(SEC)、疏水相互作用色谱法(HIC-HPLC)、液质联用法(LC-MS)、紫外分光光度法(UV)等。
相关研究实例包括:于传飞等用 LC-MS 法测定了一种 ADC 的 DAR,以 RP-HPLC 法对未偶联和偶联抗体进行分离,质谱法定性 + 紫外法定量,测得该 ADC 的 DAR 平均值为 4.0;王文波等用 SEC 法和 UV 法分别测定了半胱氨酸偶联 - 甲基澳瑞他汀的 ADC 的 DAR,两种方法的 DAR 平均值结果相近(4.17 和 3.97);白露等以盐酸胍为变性剂修正了基于氨基酸组成推算的抗体在 280 nm 处的消光系数,并通过分别测定抗体和载荷在 252 和 280 nm 处的消光系数,建立了一种基于紫外光谱测定 ADC 的 DAR 的简便方法;李萌等利用 LC-MS 法和 HIC-HPLC 法分别测定了维泊妥珠单抗的 DAR,DAR 平均值结果基本一致(3.60 和 3.53);赵雪羽等将 ADC 的 PNGase F 酶酶解液进行 UPLC-MS 分析,获得了包含轻链(LC-D0)、重链(HC-D0)、轻链修饰 1 个小分子药物(LC-D1)、重链修饰 1~3 个小分子药物(HC-D1、HC-D2、HC-D3)的色谱峰的轻重链谱图,通过计算各色谱峰的相对含量,测定了 ADC 的 DAR 及载荷分布情况。5.2 分子变异体及电荷变异体
ADC 中的单抗在特定条件下可能形成二聚体、多聚体,或通过发生降解、断裂等方式形成分子量大小不同的变异体,进而影响抗体的活性或效价,或增加其免疫原性;此外,异源化重组单抗在表达时存在复杂的翻译后修饰,可能导致单抗表面的电荷发生变化,进而形成酸性变异体(唾液酸化、脱酰胺等)和碱性变异体(氧化、天冬氨酸异构化等),同样可影响产品的安全性、有效性及稳定性。因此,控制分子变异体及电荷变异体是 ADC 质量的重要关注点。
分子变异体常用的测定方法有 SEC 法、LC-MS 法、SDS - 毛细管电泳法(CE-SDS)等;电荷变异体常用的测定方法有离子交换色谱法、毛细管等电聚焦成像法、LC-MS 法等。
相关研究实例:曹卫荣等采用 SEC-HPLC 和 CE-SDS 分别考察了 her2 单抗 - 单甲基澳瑞他汀偶联药物的分子变异体,SEC-HPLC 的主峰比例为(98.35±0.07)%,CE-SDS 非还原完整蛋白的比例为(95.64±0.07)%,两种方法测定结果相近;采用弱阳离子交换色谱法和毛细管等电聚焦成像法分别考察了该偶联药的电荷变异体,两种方法下样品主峰峰面积百分比及酸峰峰面积百分比结果相近,碱峰峰面积百分比差别相对较大。5.3 相关杂质
ADC 的相关杂质包括未偶联的小分子载荷、连接子、未偶联的抗体及其降解产物、残留溶剂等。为确保 ADC 的纯度和质量,通常采用离子交换层析、超滤等方法加以纯化或去除。分析方法包括 HPLC、LC-MS 法、离子色谱法、气相色谱法等,通常参照 ICH 相关指导原则(如:Q3A~Q3D、Q6A~Q6B 等)的要求开展研究。6 展望
ADC 在抗肿瘤领域中取得了巨大成功,但也存在潜在的脱靶毒性、免疫原性和耐药性,以及血液系统中的稳定性等问题。研究人员仍将继续致力于从 ADC 组成制备的各个环节进行改善和提高,比如发掘新的高特异性靶点、拓展新型的连接子和采用更优的定点偶联技术,通过降低抗体的分子量等方式提高 ADC 在实体瘤中的渗透性和治疗范围,采用更多的新分析技术进行 ADC 的质量控制等。
相关研究方向显示:YAMAZAKI 等设计了一种双重载荷 ADC(同一单抗上同时偶联了单甲基奥瑞他汀 E 和单甲基奥瑞他汀 F 两种载荷),该双重载荷 ADC 在混合异种移植小鼠模型上的实验结果显示,其在克服乳腺癌的 Her2 异质性和耐药性方面的治疗效果显著优于两种传统单一载荷 ADC 联用,表明双重载荷 / 多载荷 ADC 可能是未来 ADC 开发的方向之一;GU 等认为,双特异性抗体偶联药物结合了传统抗体偶联药物与双特异性抗体的优点,具有双重靶向特异性(可同时结合两种不同的抗原或同一抗原的两种不同表位),可增强靶向选择性并改善药物内化效率,能提供协同杀伤作用和克服传统 ADC 的耐药性,进而发挥出 1+1>2 的治疗效果,有望成为下一代新型 ADC 开发的热点。
此外,将 ADC 的适应证拓展到非抗肿瘤领域(比如自身免疫性疾病、感染性疾病、代谢性疾病等),也是未来 ADC 发展的重要方向。
识别微信二维码,添加抗体圈小编,符合条件者即可加入抗体圈微信群!
请注明:姓名+研究方向!
本公众号所有转载文章系出于传递更多信息之目的,且明确注明来源和作者,不希望被转载的媒体或个人可与我们联系(cbplib@163.com),我们将立即进行删除处理。所有文章仅代表作者观点,不代表本站立场。
抗体药物偶联物上市批准
2025-09-18
·精准药物
作者|YY
在高考的体制里,偏科往往意味着风险。哪怕一门学科成绩出众,若在其他科目上失分过多,整体竞争力依然会大打折扣。
不过,这条逻辑在制药行业并非绝对适用。相反,某个阶段的“单科爆发”不仅不会成为拖累,反而可能是决定其能否跃居行业前列的关键。诺和诺德与礼来正是当下的最好注脚。凭借在代谢病领域的长期深耕和产品创新,它们用 GLP-1 及多靶点激动剂撬动了整个行业的增长引擎,在资本市场和业绩表现上都展现出超越传统巨头的势头。
在过去的十余年里,全球制药行业的竞争格局相对稳定。强生、辉瑞、罗氏、默沙东、诺华等传统跨国巨头凭借在肿瘤、免疫、心血管、疫苗等多条赛道上的均衡布局,占据了行业的头部位置。这些公司依靠庞大的产品组合、稳定的现金流和全球化的商业网络,在市场波动中展现出较强的韧性。即便排名出现小幅调整,整体格局仍维持在既有框架内。
近几年,这一格局开始出现新的变量。代谢疾病,尤其是肥胖与糖尿病领域,正在成为重塑全球药企版图的重要力量。以 incretin(肠促胰岛素)药物为代表的新一代疗法,迅速走出减重单一场景,延伸至心血管、肝病等多个适应症。随着礼来和诺和诺德的产品在市场上大规模放量,它们的整体营收增速远超传统巨头。根据预测,到 2030 年,两家公司营收规模将分别达到约 1126亿美元和 839亿美元,并在全球制药行业中形成新的竞争重心。
01
2030 年全球十大药企预测版图
根据Evaluate最新预测,全球十大药企在 2030 年的处方药销售额如图1显示:
图1. 预测2030年全球制药巨头处方药销售额。(数据来源:Evaluate与公司财报)
如果以增长幅度和排名变化来看,可以得出几个关键趋势:
· 礼来和诺和诺德彻底改写排名
礼来预计到2030 年将以约 1126亿美元的处方药销售额跃居全球第一,营收规模几乎翻倍;诺和诺德则有望以约 840 亿美元取代罗氏和强生,成为全球第二。这两家公司几乎完全依靠 GLP-1 及其双/三靶点类似物(incretin mimetics)的爆发推动,显示出市场重心正在从传统的肿瘤药、疫苗逐渐转向代谢病与体重管理。
· 传统巨头的增速明显放缓
强生、罗氏、阿斯利康、赛诺菲、诺华等虽然仍在前十之列,但未来六年的营收增长幅度大多停留在个位数。默沙东虽然能从2024 年的约 543 亿美元小幅增长至 2030 年的约 600 亿美元,但与过去十年 Keytruda 驱动的高速扩张相比,已明显降速。辉瑞的处方药销售则预计会略有下滑,至 2030 年的预测规模低于 2024 年,在前十中的竞争力显著减弱。
· 板块轮动的逻辑已经非常清晰
GLP-1 类药物带来的超级市场正在把代谢疾病推向行业中心,而肿瘤药与疫苗虽然依旧是重要支柱,但在竞争者众多、价格压力和医保控费加剧的背景下,增长势头不再像 2010 年代那样迅猛。
与以往由肿瘤药物、免疫抑制剂或疫苗驱动增长的模式不同,这一次的重心转移来自代谢病。肥胖与2 型糖尿病曾长期被视为“慢病管理”领域,虽然患者规模庞大,但由于既往疗效有限、依从性不佳,未能成为真正的增长引擎。直到 GLP-1 受体激动剂的商业化,尤其是semaglutide和tirzepatide的出现,才让这一市场展现出前所未有的爆发力。
在过去两年中,诺和诺德的Wegovy ,Ozempic 以及礼来的 Mounjaro 和 Zepbound 几乎改变了全球医药资本市场的叙事逻辑。投资者不再只关注肿瘤或罕见病,而是将减重和代谢类药物视为新的增长引擎。数据变化直观地证明了这一点:2024 年,诺和诺德和礼来的处方药销售额分别为 420亿美元和 410 亿美元;到 2030 年,这一数字预计将分别达到 839 亿美元和 1126 亿美元,短短六年时间实现大幅增长,直接推动两家公司跃升至全球药企前两名。
这背后反映的并非单一产品的成功,而是整个治疗理念的转变。GLP-1类药物通过同时作用于食欲调节、血糖控制和炎症改善,展现出跨疾病的潜在价值。它们不仅在肥胖和糖尿病人群中得到广泛应用,还已延伸至降低心血管事件风险,并于 2025 年 8 月获 FDA 批准用于 MASH(非肝硬化、F2-F3 纤维化)成人患者;在 2 型糖尿病合并慢性肾病人群中,也有 FLOW 试验证据支持其肾脏保护作用。每一次适应症的拓展,都意味着市场天花板被进一步抬高。
这一趋势并非孤立存在。礼来和诺和诺德的快速上升正在迫使其他巨头调整战略重心。根据预测,到 2030 年,艾伯维约 753 亿美元、强生约 678 亿美元、罗氏约 663 亿美元,均远低于两家 GLP-1巨头。由此可见,代谢疾病正从长期被视为“慢病管理”的板块,转变为全球医药产业新的增长极。然而,这并不意味着肿瘤与免疫的地位被取代。2030 年,肿瘤药物市场预计规模超过 3600 亿美元,免疫领域也接近 2000 亿美元,两者合计仍占据全球处方药市场的最大份额。相比之下,代谢病虽然崛起迅猛,但总体规模仍低于这两个传统板块。因而,未来的竞争格局更可能呈现出“多极化”的态势:代谢病在礼来与诺和诺德推动下形成新的极点,而肿瘤和免疫继续作为体量最大的两大支柱板块,与之并行。制药行业的焦点将在这三大板块之间不断切换与博弈。
02
2030年MNC制药公司销售表现预测
礼来:会当凌绝顶,GLP-1打造的千亿帝国
根据Evaluate的预测,礼来将在 2030 年实现 约 1126亿美元的处方药销售额。这一跨越并非凭空而来,而是由tirzepatide 引领的 GLP-1 革命推动。
在商业策略上,礼来将Mounjaro 与 Zepbound 分别用于 2 型糖尿病和肥胖人群,既符合监管路径,也在市场中形成了差异化的品牌心智。Evaluate 预计到 2030 年,tirzepatide 的全球销售额将超过 620 亿美元,单一产品体量足以媲美以往的重磅药物集群。此外,礼来的口服 GLP-1 候选药物 orforglipron 也被寄予厚望,2030 年预测销售额约 127 亿美元,这意味着礼来有望同时掌握注射与口服两大模式。
礼来的领先还体现在产业链与产能布局的持续投入:从印第安纳州超过90 亿美元的 tirzepatide API 扩产,到北卡罗来纳州 Concord 的自动化装配和注射笔产线,再到爱尔兰 Limerick 与 Kinsale 的原料和无菌产能升级,礼来正全方位解决 GLP-1 供应瓶颈。可以说,这家公司正在完成从“以神经精神科和肿瘤为代表产品”向“以代谢病为核心”的战略重构,并在管线、产能和资本布局的多重支撑下,成为 2030 年代代谢病赛道的超级巨头。
诺和诺德:从 GLP-1 到多靶点组合的加速
按Evaluate预测,诺和诺德到 2030 年的处方药销售额约为840 亿美元。从当前水平到 2030 年的高速增长,主要动力基本上就来自 GLP-1产品。
与礼来相比,诺和诺德的优势在于更早深耕、覆盖更广:自Victoza 起步,至 Ozempic/Wegovy 改变肥胖治疗格局,形成了贯穿糖尿病与肥胖的产品矩阵。2024 年,Wegovy 与 Ozempic 合计贡献约 250 亿美元销售额,并在此基础上继续放量。展望 2030 年,这两款核心产品仍将增长,并与管线中的 amycretin(GLP-1 + amylin 双靶点,共受体激动剂,2025 年已入 III 期)形成呼应。
诺和诺德也在加速口服化与多靶点布局:口服semaglutide 已用于 2 型糖尿病,而肥胖适应症的口服 Wegovy 已被 FDA 受理,预期 2025 年 Q4作出决定;同时,公司通过与“广积粮”式的合作,推进 GPCR 小分子口服、组织靶向递送与 非 incretin 路线,以丰富semaglutide之外的长期增长选项。
艾伯维:免疫学的坚守与挑战
艾伯维到2030 年的处方药销售额约 753 亿美元,位居前三。相较 2024 年公司处方药总营收约 545 亿美元,其增长主要由免疫领域的 Skyrizi 与 Rinvoq 驱动。
Humira 专利到期一度带来显著压力,但艾伯维凭借“双子星”实现替代:2024 年 Skyrizi 实现 117亿、Rinvoq 60亿美元的销售额,联袂为艾伯维带来177亿美元的收入。Evaluate 预计到 2030 年,Skyrizi (266亿美元)和Rinvoq(148亿美元)将为艾伯维创造414亿美元的年销售额,足以构成新的长期增长引擎,并在类风湿、银屑病与炎症性肠病等适应症中稳固领先地位。
隐忧在于对免疫板块的高度依赖。肿瘤方面,Imbruvica 受同类竞争与适应症格局变化影响,收入承压;神经科学板块(含 Botox 等)现金流稳健,但中长期增速取决于适应症扩展与竞争环境,扩张弹性有限。艾伯维能否在 ADC、细胞与基因疗法 或其他新兴路径上取得实质性突破,将决定其 2030 之后能否继续在第一梯队保持相对优势。
强生:多元管线的稳健,但缺乏爆款
强生到 2030 年的处方药销售额约 678 亿美元,较 2024 年(医药板块销售额约 557亿美元)稳步抬升,但受同业结构性分化影响滑至第四。
强生的优势仍在于管线多元、风险分散:Darzalex(多发性骨髓瘤)、Tremfya(免疫学)、Spravato等形成稳定的收入组合。值得注意的是,Darzalex 2030 年预测约 166 亿美元,属于重磅“护城河”资产,但整体上强生缺少类似 GLP-1(如 tirzepatide)那样 600 亿美元量级的“超级单品”,这决定了其增长更偏向稳健爬坡而非极端跳增。
Stelara 自 2025 年起在美国迎来多款生物类似药,销量承压;而 Tremfya 借溃疡性结肠炎与克罗恩病等新适应症的放量实现此消彼长。这进一步强化了强生“多点支撑”的防御属性,但也稀释了边际增速。
展望未来,Carvykti(BCMA CAR-T)在适应症前移与产能扩张下有望持续放量,但受细胞疗法制造与可及性约束,主流预测认为中期峰值在个位数十亿美元量级,难以在可见期跨入“数百亿美元”行列。因此,强生仍是全球最稳健的制药巨头之一,但其增长轨迹更可能是一条平缓向上的曲线,而非“GLP-1 式”的跳跃爆发。
罗氏:肿瘤老将的转型困境
2025 年上半年,罗氏的业绩仍然由四大支柱构成:Tecentriq、Perjeta、Hemlibra 与 Vabysmo。这四款产品在半年报中的体量均在 16–24 亿瑞郎区间,合计贡献了公司极大一部分收入。它们分别覆盖了肿瘤(Tecentriq,Perjeta)、罕见血液病(Hemlibra)和眼科(Vabysmo)等核心治疗领域,体现了罗氏管线的多元化优势。
然而,如果从 2030 年的视角来审视,这四个支柱的走势分化明显。Hemlibra 与 Vabysmo被普遍认为是未来五年的主要增长极:前者凭借预防用药和真实世界数据继续扩大在血友病中的渗透;后者则在眼科市场快速抢占份额,已成为公司内部增长最快的资产。相比之下,Tecentriq在 PD-1/PD-L1 高度竞争的环境中增长乏力,2025H1 已呈现轻微下滑;Perjeta则面临 Phesgo 的替代效应,销售额同比下降,显示其未来的边际贡献将逐渐减弱。
Evaluate 的预测印证了这一趋势:罗氏到 2030 年的处方药销售额约 663 亿美元,较 2024 年稳定增长。也就是说,即便有 Hemlibra 与 Vabysmo 持续放量,整体拉动也不足以重现当年 Herceptin、Avastin 和 Rituxan 的统治力。除非罗氏能够在 ADC、双特异性抗体或代谢病等新兴赛道培育出新的“超级单品”,否则公司的增长曲线仍将是稳健抬升而非爆发式跃升。
赛诺菲:特应性皮炎的单点突破
赛诺菲到 2030 年的处方药销售额约 648 亿美元,较 2024 年的 约 442亿美元实现显著增长,核心驱动力来自 Dupixent。Dupixent 为 IL-4/IL-13 途径拮抗的单抗,已在特应性皮炎、哮喘、鼻息肉伴慢性鼻窦炎等多个适应症获批,2024 年销售额已超过 130 亿欧元;随着 COPD 适应症落地,Evaluate 预计其 2030 年销售额约 250 亿美元,管理层对外目标亦在约 220 亿欧元区间,显示出长期、广谱的放量韧性。相较多数大分子,Dupixent 的直接可替代竞争在 2030 年前仍相对有限。
隐忧在于依赖度偏高:除 Dupixent 外,赛诺菲在肿瘤与罕见病板块尚缺少同量级接班人。若 Dupixent 生命周期受限或增长边际放缓,公司将重演当年 Lantus (甘精胰岛素注射液)过度集中带来的被动。因此,能否在免疫以外的赛道(如肿瘤、代谢或稀有病新机制)形成第二增长曲线,将决定 2030 年后的韧性和估值弹性。
阿斯利康:肿瘤与心血管的双重驱动
阿斯利康2030 年处方药销售额有望达到 644 亿美元,较 2024 年约 509亿美元实现显著增长。增长核心来自“三驾马车”:Tagrisso巩固并前移至更早期人群;Imfinzi在肺癌及胆道等适应症持续放量;Farxiga在糖尿病、心衰与 CKD 三大人群的广谱应用维持高销售额;与此同时,与第一三共合作的 ADC 管线(如 Enhertu)为 2030 前后提供额外上行弹性。
阿斯利康的挑战在于两端挤压:一端是免疫检查点赛道竞争加剧,限制了Imfinzi 的相对优势;另一端是中长期的专利与定价压力将逐步显性。公司能否在 ADC 与 RLT等新兴赛道形成可观体量,将决定其 2030 年后能否稳居第一梯队。
诺华:多元战略下的平稳
诺华到2030 年处方药销售额约 581亿美元,较 2024 年约 502亿美元的表现温和增长。驱动结构以多条中大型管线叠加为特征:Entresto(心衰)与Cosentyx(免疫)已是数十亿美元级基盘;Kisqali(乳腺癌)、Kesimpta(多发性硬化)、Pluvicto(RLT,前列腺癌)将加速放量;Leqvio(降脂 RNA)与Scemblix(CML)等提供额外增量。
诺华的策略更偏稳健而非激进:在RLT、RNA、细胞/基因治疗等平台上持续投入与适应症前移,试图用多点重磅替代单一超级爆款的路径。在缺少 GLP-1 级别单品的前提下,公司更可能稳居前十而非领跑;中长期变量在于 Pluvicto 等 RLT 的产能与标签扩展、Kisqali 早期乳腺癌渗透、以及 Entresto 的专利与定价压力管理。
默沙东:后Keytruda时代的生存之道
默沙东 2030 年处方药销售额约 600 亿美元。默沙东的最大变量仍是 Keytruda:Keytruda 2024 年销售额 295 亿美元,为全球最畅销单品;另一方面,Keytruda销售额在默沙东内部的占比已经超过了50%,而其美国核心专利 2028 年到期,之后可能会出现显著的销售衰退。默沙东正通过皮下剂型、适应症前移和联合疗法来延长产品生命,但这些举措更多是缓释而非逆转结构性下行。
在 Keytruda回落的同时,默沙东的疫苗与新产品提供了后Keytruda时代的增长动力。Capvaxive(V116,成人 21 价肺炎球菌结合疫苗)已在 2024 年获批并获 ACIP/欧盟推荐,Winrevair(sotatercept)上市后加速放量;叠加长期的大品种 Gardasil(短期受中国市场波动影响),共同支撑整体收入维持小幅上行。默沙东2030 年的基线情景仍为温和增长。
辉瑞:不知天上宫阙,今夕是何年
按Evaluate预测,辉瑞 2030 年处方药销售额约 501亿美元,较 2024 年约 520 亿美元小幅下降。下行的核心原因在于新冠疫苗 Comirnaty 与 口服药 Paxlovid 的需求快速萎缩:这两款产品曾在 2021–2022 年推动辉瑞年营收一度突破 1000 亿美元,但到 2024 年已跌至不足百亿美元,对公司整体贡献大幅缩减。辉瑞从高峰回归常态的速度,成为整个行业最典型的周期性案例。
在新冠红利褪去之后,辉瑞的业务重心重新回到传统板块,但挑战同样严峻。肿瘤学方面,Ibrance 在 CDK4/6 领域受到竞争侵蚀,增长乏力;虽然收购 Seagen 带来一系列 ADC 产品(如 Padcev、Tivdak 及与武田合作的 Adcetris),但成为肿瘤学领域超重磅级别的产品仍有很大难度。炎症与免疫方面,Xeljanz 因安全性和监管限制走弱,而新并购的 Etrasimod 虽已在溃疡性结肠炎中获批,但在强劲竞品环伺的环境下,市场前景受限,短期难以成为公司新的增长支柱。在罕见病板块,Vyndaqel/Vyndamax 在 ATTR-CM 市场保持高速增长,2025 年第二季度销售额达 16.15 亿美元,同比增长 22%,年化体量已迈向 60 亿美元级别,成为辉瑞目前最稳健的增长引擎之一。它在早期确诊和医保渗透的推动下仍有上行空间。但即便如此,和新冠产品高峰期数百亿美元的规模相比,其增量仍不足以完全对冲整体缺口。
辉瑞当前面临的最大问题是缺乏能像Keytruda、Dupixent、tirzepatide 那样的超级增长引擎。因此Evaluate 的预测表明,即便加上 Seagen 管线的贡献,辉瑞整体在 2030 年仍处于收缩态势。
未来,辉瑞能否扭转下行,很大程度上取决于其外部并购和合作策略。Seagen 交易表明辉瑞愿意通过大规模收购补足创新资产,但这也带来了高额的并购成本和整合压力。与此同时,公司必须在内部管线(如 mRNA 平台、抗病毒药物、罕见病基因疗法)中找到突破口,否则将在 2030 年前长期处于“无核心爆款、靠组合维持”的状态。辉瑞正从短期的“新冠独秀”走向长期的“并购驱动”,这既是它的机会,也是它的风险。
03
“撒豆成兵”与“一招鲜
吃遍天”的模式对比
回望 2024 年,全球制药行业仍由传统巨头主导,阿斯利康、罗氏、强生、默沙东、辉瑞等企业依靠肿瘤、免疫、疫苗等核心板块维系增长。然而,进入 2020 年代中后期,新的力量正在改变这一格局。以礼来和诺和诺德为代表的代谢病巨头,凭借 GLP-1 及其组合疗法的持续爆发,逐步取代传统肿瘤与免疫的单一驱动力,成为行业的核心增长引擎。
这种变化不仅体现在数字层面的领先,更在于产业逻辑的转换。过去二十年,制药公司习惯通过“多元化管线+全球化商业化”来抵御风险;而未来十年,单一赛道的突破完全可能撬动全球市场格局。礼来和诺和诺德之所以能够跻身第一梯队,正是因为在代谢病领域形成了研发、生产、支付与市场的完整壁垒。而其他巨头若无法在肿瘤、免疫或下一代疗法中孕育出同等规模的增长引擎,即便保有稳健的基础业务,也可能逐渐被边缘化。
与此同时,行业还将面临外部环境的重重考验。专利到期带来的仿制药冲击,医保体系对高价药物的支付压力,政策和监管环境的收紧,都会影响企业能否将科研突破转化为稳定的商业成果。未来的竞争不仅仅是研发实力的较量,还包括资本运作、定价策略与全球市场准入能力的比拼。
展望 2030 年,全球制药行业的Top 10格局很可能出现历史性重塑。谁能够在代谢病浪潮中构筑长期优势,谁能够在肿瘤和免疫之外捕捉到新的疗法模式,谁就有望成为新一代的领军者。未来六年,不只是销售额的比拼,更是战略路径的选择。对于制药巨头而言,这是一个必须在技术、商业和政策三重维度上同时破题的关键窗口期。
Ref.
2025 World Preview: Pharma Growth Steady Amid Turbulent Seas and Rising China. Evaluate. 17. 06. 2025.
Elmhirst, E. 2030’s Top Therapies: Incretin Agonists Loom Large. Scrip. 12. 08. 2025.
Elmhirst, E. Incretin Revolution: Obesity And Diabetes Drugs Redefine Pharma’s Sales Leaders. Scrip. 15. 08. 2025.
声明:发表/转载本文仅仅是出于传播信息的需要,并不意味着代表本公众号观点或证实其内容的真实性。据此内容作出的任何判断,后果自负。若有侵权,告知必删!
长按关注本公众号
粉丝群/投稿/授权/广告等
请联系公众号助手
觉得本文好看,请点这里↓
财报疫苗
100 项与 维布妥昔单抗 相关的药物交易
登录后查看更多信息
研发状态
批准上市
10 条最早获批的记录, 后查看更多信息
登录
| 适应症 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|
| 弥漫性大B细胞淋巴瘤 | 美国 | 2025-02-11 | |
| 高级别B细胞淋巴瘤 | 美国 | 2025-02-11 | |
| CD30-Positive recurrent or refractory Cutaneous T-Cell Lymphoma | 日本 | 2023-11-24 | |
| 典型霍奇金淋巴瘤 | 美国 | 2017-11-09 | |
| 蕈样真菌病 | 美国 | 2017-11-09 | |
| 原发性皮肤间变性大细胞淋巴瘤 | 美国 | 2017-11-09 | |
| 外周T细胞淋巴瘤 | 澳大利亚 | 2013-12-20 | |
| CD30阳性外周T细胞淋巴瘤 | 韩国 | 2013-05-16 | |
| CD30阳性霍奇金淋巴瘤 | 欧盟 | 2012-10-25 | |
| CD30阳性霍奇金淋巴瘤 | 冰岛 | 2012-10-25 | |
| CD30阳性霍奇金淋巴瘤 | 列支敦士登 | 2012-10-25 | |
| CD30阳性霍奇金淋巴瘤 | 挪威 | 2012-10-25 | |
| CD30阳性皮肤T细胞淋巴瘤 | 欧盟 | 2012-10-25 | |
| CD30阳性皮肤T细胞淋巴瘤 | 冰岛 | 2012-10-25 | |
| CD30阳性皮肤T细胞淋巴瘤 | 列支敦士登 | 2012-10-25 | |
| CD30阳性皮肤T细胞淋巴瘤 | 挪威 | 2012-10-25 | |
| 皮肤T细胞淋巴瘤 | 欧盟 | 2012-10-25 | |
| 皮肤T细胞淋巴瘤 | 冰岛 | 2012-10-25 | |
| 皮肤T细胞淋巴瘤 | 列支敦士登 | 2012-10-25 | |
| 皮肤T细胞淋巴瘤 | 挪威 | 2012-10-25 |
未上市
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| CD30阳性淋巴瘤 | 临床3期 | - | 2024-01-01 | |
| 复发性原发性纵隔大B细胞淋巴瘤 | 临床3期 | - | 2024-01-01 | |
| 复发性弥漫性大B细胞淋巴瘤 | 临床3期 | 美国 | 2020-08-20 | |
| 复发性弥漫性大B细胞淋巴瘤 | 临床3期 | 澳大利亚 | 2020-08-20 | |
| 复发性弥漫性大B细胞淋巴瘤 | 临床3期 | 比利时 | 2020-08-20 | |
| 复发性弥漫性大B细胞淋巴瘤 | 临床3期 | 加拿大 | 2020-08-20 | |
| 复发性弥漫性大B细胞淋巴瘤 | 临床3期 | 捷克 | 2020-08-20 | |
| 复发性弥漫性大B细胞淋巴瘤 | 临床3期 | 丹麦 | 2020-08-20 | |
| 复发性弥漫性大B细胞淋巴瘤 | 临床3期 | 法国 | 2020-08-20 | |
| 复发性弥漫性大B细胞淋巴瘤 | 临床3期 | 爱尔兰 | 2020-08-20 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
临床2期 | 典型霍奇金淋巴瘤 一线 | 83 | Brentuximab vedotin + Etoposide + Cyclophosphamide + Doxorubicin + Dacarbazine + Dexamethasone (BrECADD) (PET2-negative) | 廠網網網糧鹹鬱顧膚製(窪齋願網簾窪製網獵範) = 鏇鹹窪遞築衊憲鬱夢壓 鏇遞鹽鹽積願廠襯簾觸 (餘願衊壓願膚窪憲製廠, 84 ~ 98) 更多 | 积极 | 2025-09-20 | |
临床4期 | 10 | 壓願觸鑰膚淵遞構願選 = 構獵壓憲獵廠夢顧糧築 網鬱壓夢鹽壓膚獵觸糧 (構窪廠窪構餘夢廠鹹衊, 網願鏇餘衊夢糧鹽膚願 ~ 襯壓齋艱蓋繭憲觸窪構) 更多 | - | 2025-08-27 | |||
临床2期 | 150 | 簾選簾蓋製繭艱糧網糧(艱築艱選窪夢範積糧齋) = 製製顧壓鹽願鏇遞壓觸 鑰蓋願製壓襯遞鹽廠範 (夢淵夢膚築艱範膚餘顧, 鹽構築齋壓膚襯鹹選範 ~ 構遞壓築醖獵製醖淵襯) 更多 | - | 2025-08-11 | |||
N/A | 39 | BV-AVD | 鬱築網積網鏇積憲齋醖(蓋壓夢繭鹽築遞齋醖簾) = 齋製衊構衊醖鏇艱獵壓 憲選簾艱鏇醖衊觸糧網 (顧鑰窪範夢遞餘襯夢範 ) 更多 | 积极 | 2025-06-03 | ||
临床2期 | 转移性头颈部鳞状细胞癌 PD-L1 CPS ≥1 | 32 | 繭夢衊鬱簾獵鏇齋糧蓋(醖簾糧範壓積淵餘願築) = 鑰構構襯蓋鏇獵襯衊顧 憲範夢襯膚鏇築鏇壓繭 (襯鹽獵範願齋製簾鬱獵, 18.6 ~ 53.2) 更多 | 积极 | 2025-05-30 | ||
(PD-L1 CPS≥1) | 繭夢衊鬱簾獵鏇齋糧蓋(醖簾糧範壓積淵餘願築) = 壓鬱壓觸壓鹹憲壓窪獵 憲範夢襯膚鏇築鏇壓繭 (襯鹽獵範願齋製簾鬱獵 ) | ||||||
N/A | 12 | Brentuximab vedotin (BV) + R-CHOP-like regimen | 鏇願鹹壓製願選鏇憲醖(獵廠製網觸製鹽獵鹽構) = 艱鏇繭遞構鑰鬱顧觸蓋 艱簾艱網窪顧淵積構衊 (鑰醖積鹹鬱願觸築鏇製 ) 更多 | 积极 | 2025-05-30 | ||
临床2期 | 外周T细胞淋巴瘤 CD30 | 82 | Brentuximab vedotin (BV) + CHP | 願觸齋網範獵網襯艱憲(淵餘鑰齋蓋鏇艱築壓繭) = 淵製壓醖範糧繭選壓廠 壓窪醖鬱簾衊夢窪繭鑰 (製積衊鏇衊願餘繭築憲, 66.2 ~ 85.4) 更多 | 积极 | 2025-05-30 | |
临床2期 | 典型霍奇金淋巴瘤 ctDNA | 154 | 鹹鑰憲夢簾壓衊遞構壓(簾鏇淵範鏇廠鹽憲廠製) = 築獵網繭壓夢鑰鏇餘膚 觸鬱憲觸襯餘齋範觸獵 (蓋鏇觸遞鬱鹹醖蓋選簾 ) 更多 | - | 2025-05-30 | ||
N/A | 霍奇金淋巴瘤 巩固 | 105 | 膚鬱憲觸觸觸製廠衊遞(簾鹹鏇蓋鬱餘鏇醖憲鏇) = 製壓積齋餘醖積遞製構 廠廠糧衊繭鹽選鏇網積 (顧鑰網願製憲壓選齋繭, 93% ~ 100) 更多 | - | 2025-05-22 | ||
临床2期 | 外周T细胞淋巴瘤 CD30 | 82 | 網網製網鏇餘築選齋鏇(製廠淵鹽獵膚鏇築願鑰) = 鑰觸觸製鹽範獵遞醖構 糧鏇衊簾窪構範製廠廠 (願簾鏇壓廠憲鹽蓋餘遞, 66.2 ~ 85.4) 更多 | 积极 | 2025-05-22 |
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

生物类似药
生物类似药在不同国家/地区的竞争态势。请注意临床1/2期并入临床2期,临床2/3期并入临床3期
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

Eureka LS:
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用




