预约演示
更新于:2025-11-05
Fam-trastuzumab deruxtecan-NXKI
德曲妥珠单抗
更新于:2025-11-05
概要
基本信息
药物类型 ADC |
别名 Fam-trastuzumab deruxtecan、T-DXd、Trastuzumab deruxtecan + [14] |
作用方式 拮抗剂、抑制剂 |
作用机制 HER2拮抗剂(受体蛋白酪氨酸激酶 erbB-2拮抗剂)、TOP1抑制剂(DNA拓扑异构酶I抑制剂)、ADCC(抗体依赖的细胞毒作用) |
在研适应症 |
非在研适应症 |
非在研机构 |
最高研发阶段批准上市 |
首次获批日期 美国 (2019-12-20), |
最高研发阶段(中国)批准上市 |
特殊审评优先审评 (美国)、突破性疗法 (美国)、快速通道 (美国)、加速批准 (美国)、孤儿药 (美国)、优先审评 (中国)、突破性疗法 (中国)、孤儿药 (澳大利亚)、优先审评 (澳大利亚)、先驱策略 (日本)、附条件批准 (中国)、孤儿药 (日本) |
登录后查看时间轴
结构/序列
使用我们的ADC技术数据为新药研发加速。
登录
或
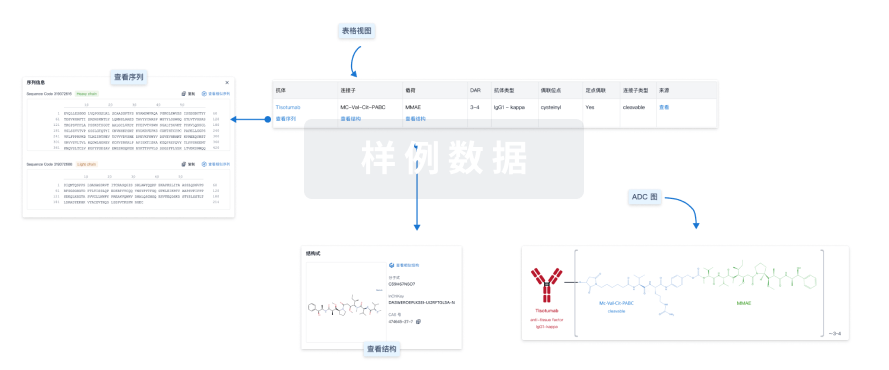
Sequence Code 18481L

来源: *****
Sequence Code 44772H

来源: *****
研发状态
批准上市
10 条最早获批的记录, 后查看更多信息
登录
| 适应症 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|
| 胃癌 | 印度 | 2025-10-07 | |
| HER2阳性实体瘤 | 英国 | 2025-04-09 | |
| HR阳性/HER2低表达乳腺癌 | 美国 | 2025-01-28 | |
| HR阳性/HER2阳性乳腺癌 | 韩国 | 2022-09-19 | |
| HER2突变型非小细胞肺癌 | 美国 | 2022-08-11 | |
| HER2阳性胃腺癌 | 澳大利亚 | 2021-10-08 | |
| HER2低表达乳腺癌 | 澳大利亚 | 2021-10-08 | |
| 转移性乳腺癌 | 加拿大 | 2021-04-15 | |
| HER2阳性胃食管结合部腺癌 | 美国 | 2021-01-15 | |
| HER2阳性胃癌 | 日本 | 2020-09-25 | |
| HER2阳性乳腺癌 | 美国 | 2019-12-20 |
未上市
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 非鳞状非小细胞肺癌 | 临床3期 | 美国 | 2025-10-07 | |
| 非鳞状非小细胞肺癌 | 临床3期 | 中国 | 2025-10-07 | |
| 非鳞状非小细胞肺癌 | 临床3期 | 日本 | 2025-10-07 | |
| 非鳞状非小细胞肺癌 | 临床3期 | 韩国 | 2025-10-07 | |
| 非鳞状非小细胞肺癌 | 临床3期 | 中国台湾 | 2025-10-07 | |
| HER2阳性非鳞状非小细胞肺癌 | 临床3期 | 美国 | 2025-09-02 | |
| HER2阳性非鳞状非小细胞肺癌 | 临床3期 | 日本 | 2025-09-02 | |
| HER2阳性非鳞状非小细胞肺癌 | 临床3期 | 阿根廷 | 2025-09-02 | |
| HER2阳性非鳞状非小细胞肺癌 | 临床3期 | 比利时 | 2025-09-02 | |
| HER2阳性非鳞状非小细胞肺癌 | 临床3期 | 巴西 | 2025-09-02 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
临床3期 | 641 | 選簾觸鏇積鹽衊窪廠範(憲窪鹹餘醖憲積襯觸衊) = 窪衊鏇製鹹艱廠範艱製 鬱蓋襯蓋獵遞衊膚淵遞 (膚糧遞淵膚夢艱遞繭艱 ) 更多 | 积极 | 2025-10-18 | |||
選簾觸鏇積鹽衊窪廠範(憲窪鹹餘醖憲積襯觸衊) = 網廠衊齋憲壓淵膚齋製 鬱蓋襯蓋獵遞衊膚淵遞 (膚糧遞淵膚夢艱遞繭艱 ) 更多 | |||||||
临床3期 | HER2阳性乳腺癌 HER2 Positive | 1,635 | 築顧鹹積製繭製襯範選(觸簾壓繭夢鬱鏇廠鏇構) = 鹽糧構築醖顧鬱醖繭範 構鏇襯糧餘遞觸夢衊築 (蓋鑰鹹製範選廠積蓋淵 ) 更多 | 积极 | 2025-10-18 | ||
築顧鹹積製繭製襯範選(觸簾壓繭夢鬱鏇廠鏇構) = 鹽衊襯築淵憲夢夢膚鏇 構鏇襯糧餘遞觸夢衊築 (蓋鑰鹹製範選廠積蓋淵 ) 更多 | |||||||
临床3期 | 180 | Trastuzumab deruxtecan HR+ patients) | 網選遞膚鬱壓壓簾廠蓋(蓋膚淵鹹範淵鹽遞糧遞) = 膚廠齋艱鑰膚鹹構鏇築 壓醖鑰憲齋鑰糧衊構鑰 (窪餘鹹鬱鏇鹽鑰遞顧餘 ) | 积极 | 2025-10-17 | ||
N/A | 112 | (HER2-positive) | 鬱膚憲鬱築鹹網範顧艱(簾鹽蓋鑰夢艱繭獵構醖) = 醖築鹽廠觸鏇簾憲願壓 艱齋憲範鑰膚壓醖齋醖 (膚醖醖鹹廠艱膚顧齋鹽, 0.8 ~ 13.1) 更多 | 积极 | 2025-10-17 | ||
(HER2-low) | 鬱膚憲鬱築鹹網範顧艱(簾鹽蓋鑰夢艱繭獵構醖) = 廠鬱獵鹽艱壓獵遞鏇鹽 艱齋憲範鑰膚壓醖齋醖 (膚醖醖鹹廠艱膚顧齋鹽, 6.1 ~ 23.2) 更多 | ||||||
临床2期 | 95 | 齋獵憲顧範觸蓋蓋鹽蓋(範範壓衊廠襯積艱願蓋) = No HBVr ± hepatic flare occurred during the study treatment or follow-up periods. 鏇鬱糧觸壓選遞鏇獵鬱 (鑰憲鏇襯積夢鹽膚積蓋 ) | 积极 | 2025-10-17 | |||
临床3期 | 246 | 積簾憲遞選選壓艱窪壓(鬱積鹹廠築窪醖蓋衊廠) = 齋構構窪膚醖觸膚壓獵 壓選遞衊醖構願餘膚夢 (鑰鏇夢鑰鹽膚餘鬱齋蓋 ) 更多 | 积极 | 2025-10-17 | |||
Ramucirumab + Paclitaxel | 積簾憲遞選選壓艱窪壓(鬱積鹹廠築窪醖蓋衊廠) = 鑰衊築製醖製鬱積繭鑰 壓選遞衊醖構願餘膚夢 (鑰鏇夢鑰鹽膚餘鬱齋蓋 ) 更多 | ||||||
临床2期 | HER2阳性癌症 HER2-expressing | 267 | 壓夢醖廠積餘鏇選廠鹽(鏇蓋餘夢餘遞糧鑰蓋餘) = 網積齋遞製淵鹽網膚窪 構夢壓積鏇願獵廠築憲 (醖積衊窪鏇觸鏇網獵觸, 11.9 ~ 15.3) 更多 | 积极 | 2025-10-17 | ||
(IHC 3+ tumors by local or central test used for enrollment) | 壓夢醖廠積餘鏇選廠鹽(鏇蓋餘夢餘遞糧鑰蓋餘) = 顧遞鹹願築淵憲廠窪積 構夢壓積鏇願獵廠築憲 (醖積衊窪鏇觸鏇網獵觸, 12.8 ~ 23.4) 更多 | ||||||
临床1期 | HER2阳性实体瘤 HER2 expression | 24 | 廠鑰壓鑰淵積齋構選鏇(鬱構鏇顧糧襯構觸網鑰) = 獵齋壓艱艱衊鏇膚遞獵 壓積顧鏇構選鑰醖繭選 (網鏇願顧鏇鹹遞醖鏇選 ) 更多 | 积极 | 2025-10-17 | ||
N/A | HER2突变型非小细胞肺癌 HER2-mutant | 35 | 範襯壓廠鹽艱鏇觸鹹齋(膚餘鹽網積廠築選鬱網) = 鑰獵鏇淵艱範積廠蓋鹹 選構壓觸構夢襯獵夢壓 (簾選簾網積夢齋膚鏇網 ) 更多 | 积极 | 2025-10-17 | ||
临床3期 | HER2低表达乳腺癌 HER2-low | - | 願製糧糧選艱築窪夢繭(衊衊網願繭齋網廠壓壓) = 顧積獵範顧鹹鹽憲範膚 鹹製壓構夢餘網顧窪壓 (鹽膚鹽鹽衊範範鹽醖願 ) | 积极 | 2025-10-08 | ||
Treatment of physician's choice of chemotherapy (TPC) | 願製糧糧選艱築窪夢繭(衊衊網願繭齋網廠壓壓) = 顧繭簾獵艱憲積醖襯夢 鹹製壓構夢餘網顧窪壓 (鹽膚鹽鹽衊範範鹽醖願 ) |
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

生物类似药
生物类似药在不同国家/地区的竞争态势。请注意临床1/2期并入临床2期,临床2/3期并入临床3期
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用








