预约演示
更新于:2025-05-16
Phenytoin
苯妥英
更新于:2025-05-16
概要
基本信息
原研机构 |
非在研机构 |
权益机构- |
最高研发阶段批准上市 |
最高研发阶段(中国)- |
特殊审评- |
登录后查看时间轴
结构/序列
分子式C15H12N2O2 |
InChIKeyCXOFVDLJLONNDW-UHFFFAOYSA-N |
CAS号57-41-0 |
关联
122
项与 苯妥英 相关的临床试验NCT06942468
Efficacy and Safety of Topical Nano Preparation of Phenytoin Plus Fractional CO2 Laser Versus Fractional CO2 Laser Alone for Treatment of Atrophic Post Acne Scars: A Randomized Split Face Study.
To evaluate the efficacy and safety of topical phenytoin spanlastics as an adjuvant therapy in treatment of atrophic post acne scars.
To compare the efficacy of topical phenytoin spanlastics combined with fractional co2 laser versus fractional co2 laser alone in treatment of atrophic post acne scars.
To compare the efficacy of topical phenytoin spanlastics combined with fractional co2 laser versus fractional co2 laser alone in treatment of atrophic post acne scars.
开始日期2025-07-01 |
申办/合作机构 |
NCT06906809
A Phase 1, Open-label, Fixed-sequence, Crossover Study to Investigate the Effect of Coadministration of the CYP3A Inducer Phenytoin and the CYP3A Inhibitor Itraconazole on the Pharmacokinetics of BGB-16673 in Healthy Participants
The purpose of this study is to investigate the effect of coadministration of phenytoin or itraconazole on the pharmacokinetics of BGB-16673 in healthy participants.
开始日期2025-04-03 |
申办/合作机构 |
NCT06903715
An Open-Label, Phase 1 Study to Characterize the Effects of Phenytoin on the Pharmacokinetics of Belzutifan in Healthy Adult Participants
Researchers designed belzutifan, the study medicine, to treat certain kinds of cancer.
The goal of this study is to learn what happens to belzutifan in a healthy person's body over time when taken, by mouth, as a tablet. Researchers will learn what happens when belzutifan is taken alone and when it is taken after several days of treatment with phenytoin.
The goal of this study is to learn what happens to belzutifan in a healthy person's body over time when taken, by mouth, as a tablet. Researchers will learn what happens when belzutifan is taken alone and when it is taken after several days of treatment with phenytoin.
开始日期2025-03-28 |
申办/合作机构 |
100 项与 苯妥英 相关的临床结果
登录后查看更多信息
100 项与 苯妥英 相关的转化医学
登录后查看更多信息
100 项与 苯妥英 相关的专利(医药)
登录后查看更多信息
23,294
项与 苯妥英 相关的文献(医药)2025-10-01·SPECTROCHIMICA ACTA PART A-MOLECULAR AND BIOMOLECULAR SPECTROSCOPY
Sensing of cyanide ion by tweaking the proton transfer
Article
作者: Brahma, Mongoli ; Mathew, Sam P ; Das, Minati ; Jaganathan, Bithiah Grace ; Krishnamoorthy, G ; Kanungo, Arup Das
The highly efficient sensing of cyanide ion was attained using a chemosensor, 2-(4'-diethylamino-2'-hydroxyphenyl)-1H-imidazo-[4,5-b]pyridine (DHP) by tuning its excited state intramolecular proton transfer to intermolecular proton transfer. Cyanide ion turns the weak blue emission of the chemosensor to bright cyan luminescence. The fluorescence is enhanced by 16 times in the presence of cyanide ion. The detection limit of CN- ion by DHP is 0.02 μM in acetonitrile. It is reversible, and the reusability was also tested for several cycles. A molecular logic gate was also constructed. The chemosensing was further tweaked by the medium. In addition, DHP has been practically applied for cyanide detection using paper strips, different water samples and sensing and imaging of cyanide ion in MDA-MB 231 breast cancer cells. To substantiate the mechanism of sensing 1H-NMR titration, density functional theory (DFT) and time dependent density functional theory (TD-DFT) calculations were employed. The transition state structures and visualization of non-covalent interactions (NCI) from reduced density gradient (RDG) and scatter plots support the proposed mechanism.
2025-08-01·Current Drug Safety
Carbamazepine-induced Stevens-Johnson Syndrome: A Case Report with Review of the Literature
Article
作者: S, Nirenjen ; T., Tamilanban ; Manavalan, Mohankumar ; Raja, Sangeetha ; Subramanian, Arunkumar ; Haitharali, Rajamohamed ; Gnanasekaran, Sabariakilesh ; Dhanasekaran, Sivaraman
Background::
Stevens-Johnson Syndrome (SJS) is an infrequent yet severe mucocutaneous
reaction that involves less than 10% of the Body Surface Area (BSA). It is predominantly
induced by certain medications, including anticonvulsants (e.g., Lamotrigine, Carbamazepine,
Phenytoin, Phenobarbitone), Allopurinol at doses above 100 mg per day, and sulphonamides
(e.g., Cotrimoxazole, Sulfasalazine). Genetic predispositions, particularly the presence of the
HLA-B*1502 allele, significantly increase the risk of developing SJS. This case report discusses
a unique presentation of SJS in a young female patient, emphasizing the critical need for genetic
screening and careful monitoring when prescribing Carbamazepine, especially in populations at
higher genetic risk.
Case Presentation::
A 19-year-old female patient, who had been on Phenytoin and Sodium
Valproate for epilepsy management over the past year, was newly prescribed Carbamazepine.
Within a week of initiating Carbamazepine, the patient experienced a seizure, followed by the
sudden onset of fever, painful sores, and blisters covering the upper body, along with mucous
discharge from both eyes. These symptoms rapidly worsened. Based on clinical presentations
and the extent of epidermal detachment, the patient was diagnosed with SJS. The severity and
mortality risks were assessed using the SCORTEN score. Therapeutic interventions included intravenous
Ranitidine, Ondansetron, Paracetamol, Midazolam, Levetiracetam, and Dexamethasone,
along with oral Fluconazole, Chlorpheniramine tablets, and Ciprofloxacin eye drops.
The patient showed significant improvement and was discharged after fourteen days with followup
advice.
Conclusion::
This case underscores the critical importance of performing genetic testing for the
HLA-B*1502 allele and conducting baseline blood tests before initiating Carbamazepine therapy.
Such precautionary measures can significantly mitigate the risk of severe adverse reactions
like SJS. This report adds to the scientific literature by highlighting the potential dangers associated
with anticonvulsant therapies and the necessity for personalized medicine approaches in
preventing life-threatening conditions. The main takeaway is the pivotal role of genetic screening
and vigilant monitoring in the management of patients requiring anticonvulsant medications to
prevent serious adverse reactions.
2025-08-01·JOURNAL OF PHARMACEUTICAL AND BIOMEDICAL ANALYSIS
Utilizing copper-doped graphene quantum dots as a fluorescent sensor for determination of carbamazepine in exhaled breath condensate
Article
作者: Jouyban-Gharamaleki, Vahid ; Rahimpour, Elaheh ; Khoubnasabjafari, Maryam ; Keyvani, Aylar ; Jouyban, Abolghasem ; Soleymani, Jafar
Carbamazepine is a versatile pharmaceutical used mainly for epilepsy and mood stabilization, but like all medications, it requires careful monitoring due to its potential side effects and interactions. In the current work, a fluorescent nanosensor was developed for the carbamazepine determination in the exhaled breath condensate (EBC). The nanosensor consists of graphene quantum dots doped with copper (Cu-doped GQDs). The presence of carbamazepine significantly decreased the fluorescence of Cu-doped GQDs, resulting in a quenching effect within a concentration range of 0.02-2.0 µg/mL, which occurs due to dynamic, rather than static, quenching according to Stern-Volmer plot. The relative standard deviations for intra-day and inter-day analysis of carbamazepine were 1.8 % and 4.8 %, respectively. The validated sensor was effectively employed for carbamazepine analysis in EBC of patients receiving the drug.
151
项与 苯妥英 相关的新闻(医药)2025-04-29
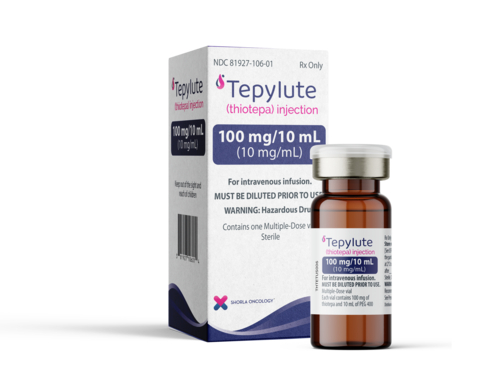
CAMBRIDGE, Mass.--(BUSINESS WIRE)--Shorla Oncology (‘Shorla’), a U.S.-Ireland specialty pharmaceutical company, announced today that the U.S. Food and Drug Administration has granted approval for 100 mg/10mL multi-dose vial of TEPYLUTE, a ready-to-dilute formulation of thiotepa to treat breast and ovarian cancer, that eliminates the need for reconstitution and may reduce preparation time and errors offering more scheduling flexibility for their patients.
Shorla Oncology Announces FDA Approval of TEPYLUTE® 100mg, First and Only Ready-to-Dilute Multi-Dose Vial of Thiotepa to Treat Breast and Ovarian Cancer and Commercial Launch of TEPYLUTE 15mg and 100mg Vials in the U.S.
“We are pleased to offer another viable treatment option for patients with breast and ovarian cancer,” said Sharon Cunningham, Chief Executive Officer and Co-Founder of Shorla Oncology. “Once opened, our 100mg vial of TEPYLUTE is stable for 14 days when properly stored, giving providers the flexibility they need when preparing and administering this very important treatment.
TEPYLUTE is a ready to dilute formulation of a well-established, standard of care oncology drug thiotepa that has been manufactured as freeze-dried powder since the 1950s.
“This is a huge win for providers because TEPYLUTE avoids the need for complicated and time-consuming reconstitution,” said Orlaith Ryan, Chief Technical Officer and Co-Founder of Shorla Oncology.
We are excited to bring TEPYLUTE to the US Market. It provides consistent dosing accuracy and allows for “just in time” preparation, which benefits everyone, especially patients.” said Rayna Herman, Chief Commercial Officer, Shorla Oncology.
The American Cancer Society estimates that more than 300,000 women will be diagnosed with breast cancer in the U.S in 2025.2 About 20,890 women will be diagnosed with ovarian cancer in the U.S. in 2025.3
About Shorla Oncology
Shorla Oncology is a privately- held, U.S. and Ireland- based commercial stage specialty pharmaceutical company established by Sharon Cunningham and Orlaith Ryan. The company has an advanced pipeline of innovative oncology drugs for orphan and pediatric cancers. Shorla is focused on indications where existing treatments are limited, in shortage or the drug applications are inadequate for the target population. The company’s growing portfolio brings accessible, affordable and life-saving treatments to patients, delivering a major contribution to patient care. Shorla currently markets three products, Nelarabine for the treatment of T-cell leukemia, JYLAMVO™ for the treatment of acute lymphoblastic leukemia and other indications and IMKELDI for the treatment of Chronic Myeloid Leukemia, Gastrointestinal Stromal Tumors (GISTs) and other indications.
For further information, please visit www.shorlaoncology.com.
TEPYLUTE® (thiotepa) Injection for Intravenous Use
INDICATION
TEPYLUTE is an alkylating drug indicated for the treatment of adenocarcinoma of the breast or ovary.
IMPORTANT SAFETY INFORMATION
WARNING: SEVERE MYELOSUPPRESSION and CARCINOGENICITY
• TEPYLUTE may cause severe marrow suppression, and high doses may cause marrow ablation with resulting infection or bleeding. Monitor hematologic laboratory parameters.
• TEPYLUTE should be considered potentially carcinogenic in humans.
CONTRAINDICATIONS
TEPYLUTE is contraindicated in patients with severe hypersensitivity to thiotepa and in concomitant use with live or attenuated vaccines.
WARNINGS AND PRECAUTIONS
Myelosuppression : For patients receiving TEPYLUTE for treatment of adenocarcinoma of the breast or adenocarcinoma of the ovary, if the bone marrow has been compromised by prior irradiation or chemotherapy, or is recovering from chemotherapy, the risk of severe myelosuppression with TEPYLUTE may be increased. Perform periodic complete blood counts during the course of treatment with TEPYLUTE. Provide supportive care for infections, bleeding, and symptomatic anemia. Inform patients of the possibility of developing low blood cell counts and the need for hematopoietic progenitor cell infusion. Instruct patients to immediately report to their healthcare provider if bleeding or fever occurs.
Hypersensitivity: Clinically significant hypersensitivity reactions, including anaphylaxis, have occurred following administration of thiotepa. If anaphylactic or other clinically significant allergic reaction occurs, discontinue treatment with TEPYLUTE, initiate appropriate therapy, and monitor until signs and symptoms resolve. Counsel patients on the signs and symptoms of hypersensitivity and to seek immediate emergency assistance if they develop any of these signs and symptoms.
Cutaneous Toxicity: TEPYLUTE and/or its active metabolites may be excreted in part via skin in patients receiving high-dose therapy. Treatment with TEPYLUTE may cause skin discoloration, pruritus, blistering, desquamation, and peeling that may be more severe in the groin, axillae, skin folds, in the neck area, and under dressings. Instruct patients to shower or bathe with water at least twice daily through 48 hours after administration of TEPYLUTE. Change the occlusive dressing and clean the covered skin at least twice daily through 48 hours after administration of TEPYLUTE. Change bed sheets daily during treatment. Skin reactions associated with accidental exposure to TEPYLUTE may occur. Wash the skin thoroughly with soap and water in case the TEPYLUTE solution contacts the skin. Flush mucous membranes in case of TEPYLUTE contact with mucous membranes.
Concomitant Use of Live and Attenuated Vaccines: Do not administer live or attenuated viral or bacterial vaccines to a patient treated with TEPYLUTE until the immunosuppressive effects have resolved.
Hepatic Veno-Occlusive Disease: Monitor by physical examination, serum transaminases, and bilirubin, and provide supportive care to patients who develop hepatic veno-occlusive disease.
Central Nervous System Toxicity: Fatal encephalopathy has occurred in patients treated with high doses of thiotepa. Other central nervous system toxicities, such as headache, apathy, psychomotor retardation, disorientation, confusion, amnesia, hallucinations, drowsiness, somnolence, seizures, coma, inappropriate behavior, and forgetfulness have been reported to occur in a dose-dependent manner during or shortly after administration of high-dose thiotepa. Do not exceed the recommended dose of TEPYLUTE. If severe or life-threatening central nervous system toxicity occurs, discontinue administration of TEPYLUTE and provide supportive care.
Carcinogenicity: Like many alkylating agents, thiotepa has been reported to be carcinogenic when administered to laboratory animals. Carcinogenicity is shown most clearly in studies using mice, but there is some evidence of carcinogenicity in man. There is an increased risk of secondary malignancy with the use of TEPYLUTE. Inform patients that TEPYLUTE can increase the risk of secondary malignancy.
Polyethylene glycol (PEG) 400 toxicity : TEPYLUTE contains a high concentration of PEG 400. Based on findings in animals, administration of high amounts of PEG 400 may cause damage to the kidneys and liver at dosages higher than recommended. When prescribing TEPYLUTE, take into consideration the PEG 400 load from concomitant medications.
Embryo-Fetal Toxicity: Based on the mechanism of action and findings in animals, TEPYLUTE can cause fetal harm when administered to a pregnant woman. There are no adequate and well-controlled studies of TEPYLUTE in pregnant women. Thiotepa given by the intraperitoneal (IP) route was teratogenic in mice at doses ≥1 mg/kg (3.2 mg/m 2 ), approximately 8-fold less than the maximum recommended human therapeutic dose (0.8 mg/kg, 27 mg/m 2 ), based on body-surface area. Thiotepa given by the IP route was teratogenic in rats at doses ≥3 mg/kg (21 mg/m 2 ), approximately equal to the maximum recommended human therapeutic dose, based on body-surface area. Thiotepa was lethal to rabbit fetuses at a dose of 3 mg/kg (41 mg/m 2 ), approximately two times the maximum recommended human therapeutic dose based on body-surface area. Advise pregnant women and females of reproductive potential of the potential risk to the fetus. Advise females of reproductive potential to use highly effective contraception during TEPYLUTE treatment and for 6 months after therapy/the last dose. Advise females of reproductive potential to inform their healthcare provider if they are pregnant or become pregnant. Advise males with female partners of reproductive potential to use effective contraception during TEPYLUTE treatment and for 1 year after therapy/the last dose.
ADVERSE REACTIONS
The most common adverse reactions (incidence greater than 10%) are neutropenia, anemia, thrombocytopenia, elevated alanine aminotransferase, elevated aspartate aminotransferase (AST), elevated bilirubin, mucositis, cytomegalovirus infection, hemorrhage, diarrhea, hematuria, and rash.
The clinically significant adverse reactions include myelosuppression, infection, hypersensitivity, cutaneous toxicity, hepatic veno-occlusive disease, central nervous system toxicity, and carcinogenicity.
DRUG INTERACTIONS
Effect of Cytochrome CYP3A Inhibitors and Inducers: In vitro studies suggest that thiotepa is metabolized by CYP3A4 and CYP2B6 to its active metabolite triethylene phosphoramide (TEPA). Avoid coadministration of strong CYP3A4 inhibitors (e.g., itraconazole, clarithromycin, ritonavir) and strong CYP3A4 inducers (e.g., rifampin, phenytoin) with thiotepa due to the potential effects on efficacy and toxicity. Consider alternative medications with no or minimal potential to inhibit or induce CYP3A4. If concomitant use of strong CYP3A4 modulators cannot be avoided, closely monitor for adverse drug reactions.
Effect of TEPYLUTE on Cytochrome CYP2B6 Substrates: In vitro studies suggest that thiotepa inhibits CYP2B6. Thiotepa may increase the exposure of drugs that are substrates of CYP2B6 in patients; however, the clinical relevance of this in vitro interaction is unknown. The administration of thiotepa with cyclophosphamide in patients reduces the conversion of cyclophosphamide to the active metabolite, 4‑hydroxycyclophosphamide; the effect appears sequence-dependent with a greater reduction in the conversion to 4-hydroxycyclophosphamide when thiotepa is administered 1.5 hours before the intravenous administration of cyclophosphamide compared to administration of thiotepa after intravenous cyclophosphamide. The reduction in 4-hydroxycyclophosphamide levels may potentially reduce the efficacy of cyclophosphamide treatment.
USE IN SPECIFIC POPULATIONS
Pregnancy: TEPYLUTE can cause fetal harm when administered to a pregnant woman based on findings from animals and the drug’s mechanism of action. Limited available data on thiotepa use in pregnant women are insufficient to inform a drug-associated risk of major birth defects and miscarriage. In animal reproduction studies, administration of thiotepa to pregnant mice and rats during organogenesis produced teratogenic effects (neural tube defects and malformations of the skeletal system of the fetus) at doses approximately 0.125 and 1 times, respectively, the maximum recommended human daily dose on a mg/m2 basis. Thiotepa was lethal to rabbit fetuses at approximately 2 times the maximum recommended human therapeutic dose based on body-surface area. Consider the benefits and risks of TEPYLUTE for the mother and possible risks to the fetus when prescribing TEPYLUTE to a pregnant woman.
Lactation: There is no information regarding the presence of thiotepa in human milk, the effects on the breastfed infant, or the effects on milk production. Because of the potential for serious adverse reactions, including the potential for tumorigenicity shown for thiotepa in animal studies, advise patients not to breastfeed during TEPYLUTE treatment and for 1 week after therapy/the last dose.
Females and Males of Reproductive Potential:
TEPYLUTE can cause fetal harm when administered to a pregnant woman.
Pregnancy Testing - Verify the pregnancy status of females of reproductive potential before initiating TEPYLUTE therapy.
Contraception for Females - Advise females of reproductive potential to avoid pregnancy during TEPYLUTE treatment and for 6 months after therapy/the last dose. Advise females to immediately report pregnancy.
Contraception for Males - TEPYLUTE may damage spermatozoa and testicular tissue, resulting in possible genetic abnormalities. Males with female sexual partners of reproductive potential should use effective contraception during TEPYLUTE treatment and for 1 year after therapy/the last dose.
Infertility - Based on nonclinical findings, male and female fertility may be compromised by treatment with TEPYLUTE. Advise patients that TEPYLUTE can produce infertility. Inform male patients about the possibility of sperm conservation before the start of therapy.
Pediatric Use: Safety and effectiveness of TEPYLUTE in neonates have not been established. Safety and effectiveness of TEPYLUTE for the treatment of adenocarcinoma of the breast and adenocarcinoma of the ovary in pediatric patients have not been established.
Geriatric Use: Clinical studies of thiotepa for treatment of adenocarcinoma of the breast and adenocarcinoma of the ovary did not include sufficient numbers of subjects aged 65 years and over to determine whether elderly subjects respond differently from younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreasing hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Renal Impairment: In patients with moderate (creatinine clearance [CLcr] of 30 mL/min to 59 mL/min) renal impairment, decreased renal excretion may result in increased plasma levels of thiotepa and TEPA. This may result in increased toxicity. Monitor patients with moderate to severe (CLcr <30 mL/min) renal impairment for signs and symptoms of toxicity following treatment with TEPYLUTE for an extended period of time.
Hepatic Impairment: Thiotepa is extensively metabolized in the liver. Patients with moderate (bilirubin levels greater than 1.5 times to 3 times the upper limit of normal and any AST) hepatic impairment may have increased plasma levels of thiotepa. This may result in toxicity. Monitor patients with moderate to severe (bilirubin levels greater than 3 times the upper limit of normal and any AST) hepatic impairment for signs and symptoms of toxicity following treatment with TEPYLUTE for an extended period of time.
To report suspected adverse reactions, contact Shorla Oncology at 1-844-668-3940 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
For Print: Please see the accompanying full Prescribing Information, including Boxed Warning.
For Digital: Please click here for full Prescribing Information, including Boxed Warning.
All trademarks are the property of Shorla Oncology. ©SHORLA ONCOLOGY® 2025. PRO-TEP-1377-v1 04/2025
上市批准临床结果
2025-04-29
– New findings to be presented at the 2025 European Association for the Study of the Liver (EASL) Congress continue to demonstrate the effectiveness of Livdelzi (seladelpar) in reducing pruritus in people with primary biliary cholangitis (PBC) –
– Additional presentations will showcase the potential for maintained virologic response following treatment with investigational bulevirtide in people with hepatitis delta virus (HDV) –
– Presentations in hepatitis B virus (HBV) and hepatitis C virus (HCV) will underscore Gilead’s commitment to advancing scientific research and our understanding of viral hepatitis in the real-world setting –
FOSTER CITY, Calif.--(BUSINESS WIRE)-- Gilead Sciences, Inc. (Nasdaq:GILD) today announced new research to be presented at the European Association for the Study of the Liver (EASL) Congress, May 7-10, 2025, Amsterdam, furthering Gilead’s commitment to transform the lives of people living with liver disease.
New data to be presented at EASL will include a subgroup analysis from ASSURE, an ongoing open-label study, and an analysis of the Phase 3 RESPONSE trial and the open-label extension. Data will reinforce the effectiveness of Livdelzi® (seladelpar), known as Lyvdelzi® in the European Union, in reducing pruritus (chronic itch), a debilitating common symptom of primary biliary cholangitis (PBC). Moreover, the results will highlight seladelpar’s ability to deliver a sustained biochemical response regardless of prior treatment history, and as an option for a broad range of people with PBC.
“Our focus remains on addressing the areas of greatest unmet need across liver diseases,” said Dietmar Berger, MD, PhD, Chief Medical Officer, Gilead Sciences. “Our deep expertise in treating liver disease has delivered the first and only treatment for hepatitis delta and the first treatment for primary biliary cholangitis that has demonstrated statistically significant improvements across both chronic itch and markers of disease progression. Our studies continue to deliver transformational insights and therapies to help foster healthier futures for the millions of people who live with viral hepatitis and those who live with less prevalent liver conditions with remaining high unmet needs.”
Key findings from 29 accepted abstracts will include two oral presentations showcasing new data on the critical goal of maintained virologic response following treatment with 2 mg and 10 mg bulevirtide in people living with hepatitis delta virus (HDV). These findings build on the wealth of long-term data for the treatment of chronic HDV.
A late breaker presentation on the final results from the pivotal MYR301 Phase 3 study evaluating the efficacy and safety of 2 mg and 10 mg bulevirtide as monotherapy will reveal long-term response rates, including the proportion of participants with undetectable virus levels two years after treatment cessation.
In hepatitis B (HBV), Gilead will present initial results from a Phase 1a study of a novel investigational therapeutic vaccine that shows early promise towards the goal of achieving a functional cure for HBV infection. A separate analysis of real-world data showed that individuals with low-level HBV viremia still have a risk of negative events. Additionally, a retrospective analysis of participants enrolled in two Phase 3 studies evaluating Vemlidy® (tenofovir alafenamide) using a risk score for liver cancer highlights the importance of treatment to help reduce liver cancer risk.
Gilead will also showcase real-world data on hepatitis C (HCV), demonstrating the effects of direct-acting antivirals (DAA) against all genotypes of the virus across diverse geographical regions, supporting the global applicability of HCV treatment guidelines. In addition, new real-world insights will show that the choice of DAA for people living with HCV taking other medicines, such as antipsychotics, was associated with reducing the problem of drug-drug interactions.
To help raise awareness about HCV and encourage people to get screened for the disease, Gilead will again support EASL’s “Love Your Liver” Campaign at the congress.
At the EASL Congress, Gilead, in collaboration with the PBC Foundation and Friends of the PBC Foundation, will also launch “All the Feelings with PBC,” a campaign highlighting the experiences of people living with PBC and emphasizing the importance of listening to and addressing their needs in clinical practice and research. The campaign will feature paintings by Berlin-based artist, Nour Khwies, based on the physical and emotional experiences of people living with PBC: Dilek from Germany, Angela from Scotland, Joana from Portugal, and L. Marie from the United States, whose painting will be painted live at EASL.
Key Abstracts at EASL 2025:
ID
Abstract Title
PBC
THU-291
Clinical meaningful change of pruritus numeric rating scale in adults with primary biliary cholangitis with moderate-to-severe pruritus
THU-286
Seladelpar treatment of patients with primary biliary cholangitis improves the GLOBE score and predicts improved transplant-free survival
THU-294
Change in pruritus in patients with primary biliary cholangitis and moderate-to-severe pruritus: A pooled analysis from the RESPONSE and ENHANCE studies
THU-274
Efficacy and safety of seladelpar in patients previously treated with fibrates or OCA
FRI-318
Evaluation of the potential impact of seladelpar on the pharmacokinetics of midazolam, tolbutamide, simvastatin, rosuvastatin, and atorvastatin
HDV
OS-066
Predictors of undetectable hepatitis delta virus RNA at 48 weeks after end of treatment with bulevirtide monotherapy in the MYR 301 study
OS-070
Achieving undetectable hepatitis delta virus RNA at end of therapy with bulevirtide 10 mg/day with or without pegIFN is strongly associated with post-treatment virologic response in chronic hepatitis delta
LBO-004
Final results of MYR301: A randomised Phase 3 study evaluating the efficacy and safety of up to 144 weeks of bulevirtide monotherapy for chronic hepatitis delta and 96 weeks of posttreatment follow-up
HCV
WED-276
The SVR10K Hepatitis C study: Final results show 98.9% SVR in 7,000 patients treated with SOF/VEL in Asia, Latin America, Middle East, Nordics, and Southern Europe
WED-031
Healthcare resource use (HCRU) and cost impact of potential drug-drug interactions (DDIs) among HCV patients receiving Direct-Acting Antivirals (DAAs) concomitantly with antipsychotic drugs in the US
HBV
THU-246
Safety, tolerability and immunogenicity of GS-2829 and GS-6779, a novel arenaviral vectored therapeutic hepatitis B vaccine: results from a phase 1a study in healthy participants
SAT-009
Risk of advanced liver disease among individuals with hepatitis B virus infection with low level viremia compared to individuals with no evidence of hepatitis B virus infection
WED-296
Impact of long-term tenofovir-based treatment on hepatocellular carcinoma risk in patients with chronic hepatitis B using the reREACH-B score
For more information on the EASL Congress 2025 and Gilead’s presentations, please visit the congress website.
About Gilead Sciences in Liver Disease
For decades, Gilead has pioneered the way forward to improve the lives of people living with liver disease around the world. The company has helped to transform hepatitis C from a chronic condition into a curable condition. For individuals living with hepatitis B or hepatitis delta, Gilead's focus on advancing medicines drives hope that today’s research will turn into tomorrow’s cures. Beyond viral hepatitis, Gilead is working to deliver advanced treatments for people living with PBC. The commitment of Gilead does not stop there. Through ground-breaking science and collaborative partnerships, the company strives to create healthier futures for everyone living with liver disease. Gilead remains devoted to a future without liver disease.
Marketing Authorization
In July 2023, the European Commission (EC) granted full Marketing Authorization (MA) for Hepcludex® (bulevirtide) 2 mg for the treatment of adults with chronic HDV and compensated liver disease. Bulevirtide was initially granted a conditional MA from the EC in July 2020 to provide people living with HDV urgent access to treatment. Bulevirtide’s conditional MA license in the UK was converted to a full MA in August 2023, and a full MA was granted in Switzerland in February 2024. In regions where bulevirtide is not approved, including the U.S., bulevirtide 2 mg is an investigational product. In these regions, health authorities have not established the safety and efficacy of bulevirtide. Bulevirtide 10 mg is an investigational product and is not approved anywhere globally.
In February 2025, the European Commission (EC) granted conditional marketing authorization for Lyvdelzi® (seladelpar) for the treatment of PBC in combination with ursodeoxycholic acid (UDCA) in adults who have an inadequate response to UDCA alone, or as monotherapy in those unable to tolerate UDCA. Lyvdelzi provides an important treatment option for people living with PBC in the European Economic Area (EEA). Gilead is working with health authorities across Europe to ensure people living with PBC who are eligible for Lyvdelzi have access as soon as possible. Continued approval of Lyvdelzi for the approved indication will be contingent on verification and description of clinical benefit in confirmatory trial(s). Lyvdelzi has Priority Medicine (PRIME) designation in the EU, assigned to optimize the development of novel medicines that target conditions with an unmet medical need for which no treatment options exist or where they can offer a major therapeutic advantage over existing treatments, as well as well as Orphan Drug Designation for the treatment of people living with PBC.
Livdelzi® received conditional approval from the UK Medicines and Healthcare products Regulatory Agency (MHRA) in January 2025. In the U.S., Livdelzi® obtained accelerated approval for the treatment of PBC by the U.S. Food and Drug Administration (FDA) in August 2024. Livdelzi received Breakthrough Therapy Designation, as well as Orphan Drug Designation for the treatment of people living with PBC. As part of the FDA accelerated approval, Gilead has committed to AFFIRM, a long-term outcomes study, which has begun in people with compensated cirrhosis. Continued U.S. approval may be contingent upon verification of clinical benefit in confirmatory trial(s). Regulatory review for Livdelzi is underway in Canada, Australia, and Switzerland.
U.S. Indication for Livdelzi®
Livdelzi is indicated for the treatment of primary biliary cholangitis (PBC) in combination with ursodeoxycholic acid (UDCA) in adults who have had an inadequate response to UDCA, or as monotherapy in patients unable to tolerate UDCA. The FDA approved this indication under accelerated approval based on a reduction of alkaline phosphatase (ALP). Improvement in survival or prevention of liver decompensation events have not been demonstrated. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s).
Limitations of Use:
Use of Livdelzi is not recommended in patients who have or develop decompensated cirrhosis (e.g., ascites, variceal bleeding, hepatic encephalopathy).
U.S. Important Safety Information for Livdelzi
Warnings and Precautions
Fractures: Fractures occurred in 4% of Livdelzi-treated patients compared to no placebo-treated patients. Consider the risk of fracture in the care of patients treated with Livdelzi and monitor bone health according to current standards of care. Liver Test Abnormalities: Livdelzi has been associated with dose-related increases in serum transaminase (AST and ALT) levels > 3 x ULN in patients receiving 50 mg and 200 mg once daily (5x and 20x higher than the recommended dosage of 10 mg once daily). Perform baseline clinical and laboratory testing when starting Livdelzi® and monitor thereafter according to routine patient management. Interrupt treatment if the liver tests (ALT, AST, total bilirubin, and/or ALP) worsen, or if the patient develops signs and symptoms of clinical hepatitis (eg, jaundice, right upper quadrant pain, eosinophilia). Consider permanent discontinuation if liver tests worsen after restarting Livdelzi. Biliary Obstruction: Avoid use of Livdelzi in patients with complete biliary obstruction. If biliary obstruction is suspected, interrupt Livdelzi and treat as clinically indicated.
Adverse Reactions
The most common adverse reactions (≥5%) with Livdelzi were headache (8%), abdominal pain (7%), nausea (6%), abdominal distension (6%), and dizziness (5%).
Drug Interactions
OAT3 Inhibitors and Strong CYP2C9 Inhibitors: Avoid co-administration with L due to increased Livdelzi exposure. Rifampin: Monitor biochemical response (e.g., ALP and bilirubin) when patients initiate rifampin during Livdelzi treatment. Coadministration may result in delayed or suboptimal biochemical response of Livdelzi. Dual Moderate CYP2C9 and Moderate-to-Strong CYP3A4 Inhibitors and BCRP Inhibitors (e.g., cyclosporine): Monitor closely for adverse effects. Concomitant administration with Livdelzi may increase Livdelzi exposure. CYP2C9 Poor Metabolizers Using Moderate-to-Strong CYP3A4 Inhibitors: Monitor more frequently for adverse reactions as concomitant use of a moderate-to-strong CYP3A4 inhibitor in patients who are CYP2C9 poor metabolizers may increase Livdelzi exposure and risk of LIVDELZI adverse reactions. Bile Acid Sequestrants: Administer Livdelzi at least 4 hours before or 4 hours after taking a bile acid sequestrant, or at as great an interval as possible.
Pregnancy and Lactation
Pregnancy: There is insufficient data from human pregnancies exposed to Livdelzi to allow an assessment of a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Report pregnancies to Gilead Sciences, Inc., at 1-800-445-3235. Lactation: There is no data on the presence of Livdelzi in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Livdelzi and any potential adverse effects on the breastfed infant from Livdelzi.
U.S. Indication for Vemlidy®
VEMLIDY is indicated for the treatment of chronic hepatitis B virus infection in adults and pediatric patients six years of age and older and weighing at least 25 kg with compensated liver disease.
U.S. Important Safety Information for and Indication for the Use of Vemlidy
BOXED WARNING: POSTTREATMENT SEVERE ACUTE EXACERBATION OF HEPATITIS B
Discontinuation of anti-hepatitis B therapy, including VEMLIDY, may result in severe acute exacerbations of hepatitis B. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who discontinue anti-hepatitis B therapy, including VEMLIDY. If appropriate, resumption of anti-hepatitis B therapy may be warranted.
Warnings and Precautions
Risk of Development of HIV-1 Resistance in HBV/HIV-1 Coinfected Patients:Due to this risk, VEMLIDY alone should not be used for the treatment of HIV-1 infection. Safety and efficacy of VEMLIDY have not been established in HBV/HIV-1 coinfected patients. HIV antibody testing should be offered to all HBV-infected patients before initiating therapy with VEMLIDY, and if positive, an appropriate antiretroviral combination regimen that is recommended for HBV/HIV-1 coinfected patients should be used. New Onset or Worsening Renal Impairment:Postmarketing cases of renal impairment, including acute renal failure, proximal renal tubulopathy (PRT), and Fanconi syndrome, have been reported with TAF-containing products. Patients with impaired renal function and/or taking nephrotoxic agents (including NSAIDs) are at increased risk of renal-related adverse reactions. Discontinue VEMLIDY in patients who develop clinically significant decreases in renal function or evidence of Fanconi syndrome. Monitor renal function in all patients – See Dosage and Administration. Lactic Acidosis and Severe Hepatomegaly with Steatosis:Fatal cases have been reported with the use of nucleoside analogs, including tenofovir disoproxil fumarate (TDF). Discontinue VEMLIDY if clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity develop, including hepatomegaly and steatosis in the absence of marked transaminase elevations.
Adverse Reactions
Most common adverse events in the week 96 pediatric population reported in ≥5% were: nasopharyngitis, headache, COVID-19, pyrexia, diarrhea, upper respiratory tract infection, cough, respiratory tract infection viral, abdominal pain upper. Overall, abdominal pain upper and metabolic nephropathy were the only study drug–related adverse events, which occurred in > 1 participant, reported in 2.3% (2/88 participants) each.
Drug Interactions
Coadministration of VEMLIDY with drugs that reduce renal function or compete for active tubular secretion may increase concentrations of tenofovir and the risk of adverse reactions. Coadministration of VEMLIDY is not recommended with the following: oxcarbazepine, phenobarbital, phenytoin, rifabutin, rifampin, rifapentine, or St. John’s wort. Such coadministration is expected to decrease the concentration of tenofovir alafenamide, reducing the therapeutic effect of VEMLIDY. Drugs that strongly affect P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) activity may lead to changes in VEMLIDY absorption. Coadministration of VEMLIDY with carbamazepine, the tenofovir alafenamide dose should be increased to two tablets once daily.
Consult the full prescribing information for VEMLIDY for more information on potentially significant drug interactions, including clinical comments.
Dosage and Administration
Testing Prior to Initiation:HIV infection. Prior to or When Initiating, and During Treatment:On a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose, and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorus. Dosage:1 tablet taken once daily with food. Renal Impairment:Not recommended in patients with end-stage renal disease (ESRD; eCrCl <15 mL/min) who are not receiving chronic hemodialysis; in patients on chronic hemodialysis, on hemodialysis days, administer VEMLIDY after completion of hemodialysis treatment. No data are available to make dose recommendations in pediatric patients with renal impairment. Hepatic Impairment:Not recommended in patients with decompensated (Child-Pugh B or C) hepatic impairment.
About Gilead Sciences
Gilead Sciences, Inc. is a biopharmaceutical company that has pursued and achieved breakthroughs in medicine for more than three decades, with the goal of creating a healthier world for all people. The company is committed to advancing innovative medicines to prevent and treat life-threatening diseases, including HIV, viral hepatitis, COVID-19, cancer and inflammation. Gilead operates in more than 35 countries worldwide, with headquarters in Foster City, California.
Forward-Looking Statements
This press release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that are subject to risks, uncertainties and other factors, including Gilead’s ability to initiate, progress or complete clinical trials within currently anticipated timelines or at all, and the possibility of unfavorable results from ongoing or additional clinical trials, including those involving Lyvdelzi®/Livdelzi® (seladelpar), Vemlidy®, bulevirtide, sofosbuvir, velpatasvir, GS-2829 and GS-6779 (such as the ASSURE, MYR301, RESPONSE, SVR10K and any confirmatory studies); uncertainties relating to regulatory applications and related filing and approval timelines, including additional pending and potential applications for seladelpar; the risk that any regulatory approvals, if granted, may be subject to significant limitations on use or subject to withdrawal or other adverse actions by the applicable regulatory authority; uncertainties regarding Gilead’s ability to coordinate access to seladelpar in a timely manner or at all; the risk that physicians may not see the benefits of prescribing seladelpar for treatment of PBC; and any assumptions underlying any of the foregoing. These and other risks, uncertainties and factors are described in detail in Gilead’s Annual Report on Form 10-K for the year ended December 31, 2024, as filed with the U.S. Securities and Exchange Commission. These risks, uncertainties and other factors could cause actual results to differ materially from those referred to in the forward-looking statements. All statements other than statements of historical fact are statements that could be deemed forward-looking statements. The reader is cautioned that any such forward-looking statements are not guarantees of future performance and involve risks and uncertainties and is cautioned not to place undue reliance on these forward-looking statements. All forward-looking statements are based on information currently available to Gilead, and Gilead assumes no obligation and disclaims any intent to update any such forward-looking statements.
Hepcludex, Lyvdelzi, Livdelzi, Vemlidy, Gilead and the Gilead logo are registered trademarks of Gilead Sciences, Inc., or its related companies.
Full Prescribing Information for Livdelzi® and Vemlidy® are available at www.gilead.com .
For more information about Gilead, please visit the company’s website at www.gilead.com , follow Gilead on X/Twitter (@Gilead Sciences) and LinkedIn (@Gilead-Sciences).
Blair Baumwell, Media public_affairs@gilead.com
Jacquie Ross, Investors investor_relations@gilead.com
临床结果突破性疗法上市批准临床3期临床2期
2025-03-12
March 12, 2025 11:26 am ET
DOR/ISL demonstrated non-inferiority and a similar safety profile to comparator antiretroviral therapies in adults with virologically suppressed HIV-1
RAHWAY, N.J.--(BUSINESS WIRE)-- Merck (NYSE: MRK), known as MSD outside of the United States and Canada, today announced the presentation of positive results from two pivotal Phase 3 trials of the investigational, once-daily, oral, two-drug regimen of doravirine/islatravir [DOR/ISL (100mg/0.25mg)] in adults with HIV-1 infection that is virologically suppressed on bictegravir/emtricitabine/tenofovir alafenamidei [BIC/FTC/TAF (50mg/200mg/25mg)] in trial MK-8591A-052 ) or antiretroviral therapy [baseline antiretroviral therapy (bART)] in trial MK-8591A-051. In both trials, DOR/ISL met the primary efficacy success criterion for non-inferiority to comparator antiretroviral therapies and primary safety objectives at Week 48. The findings will be shared in late-breaking oral presentations at the 32nd Conference on Retroviruses and Opportunistic Infections (CROI) being held in San Francisco and were featured in a CROI press conference. Merck plans to begin submitting applications for marketing authorization to regulatory agencies by mid-2025.
In the double-blind trial MK-8591A-052 (Abstract #204A), results for the primary endpoint (HIV-1 RNA ≥50 copies/mL) showed that 1.5% of participants who switched to DOR/ISL had a viral load of ≥50 copies/mL at Week 48, compared to 0.6% on BIC/FTC/TAF (treatment difference 0.9%, 95% CI -1.9, 2.9). At Week 48, 91.5% of participants who switched to DOR/ISL maintained viral suppression (HIV-1 RNA <50 copies/mL) compared to 94.2% of participants who continued receiving BIC/FTC/TAF (treatment difference -2.6%, 95% CI -7.1, 2.6; secondary endpoint).
In the open-label trial MK-8591A-051 (Abstract #204B), results for the primary endpoint (HIV-1 RNA ≥50 copies/mL) showed that 1.4% of participants who received DOR/ISL had a viral load of ≥50 copies/mL at Week 48, compared to 4.9% on bART (treatment difference -3.6%, 95% CI -7.8, -0.8. At Week 48, 95.6% of participants who switched to DOR/ISL maintained viral suppression (HIV-1 RNA <50 copies/mL) compared to 91.9% of participants who continued on bART (treatment difference 3.7%, 95% CI -0.3, 8.9; secondary endpoint).
Across both trials, the safety profile of DOR/ISL was generally comparable to the comparator antiretroviral regimens, including BIC/FTC/TAF in MK-8591A-052. At Week 48, the mean percent change in total lymphocyte and CD4 counts were similar for DOR/ISL and comparator regimens. No treatment-emergent resistance to DOR or ISL was observed in either trial.
“Despite the availability of multiple daily antiretroviral therapies, the needs of people living with HIV are evolving. Many people living with HIV are older and also managing comorbidities, making it important to have daily treatment options that can help meet each person’s unique health needs,” said Professor Chloe Orkin, Dean for Healthcare Transformation, Queen Mary University of London, United Kingdom. “I’m excited to see that DOR/ISL has potential as a new daily treatment option for people living with HIV who may benefit from this two-drug regimen.”
Islatravir, Merck’s investigational nucleoside reverse transcriptase translocation inhibitor (NRTTI), blocks HIV-1 replication by multiple mechanisms including inhibition of reverse transcriptase translocation resulting in immediate chain termination and delayed chain termination from structural changes induced in the viral DNA.
“We are excited that DOR/ISL is the first two-drug regimen without an integrase inhibitor to demonstrate comparable efficacy and safety to the three-drug InSTI-based regimen, BIC/FTC/TAF, in a Phase 3 pivotal trial,” said Dr. Eliav Barr, senior vice president, head of global clinical development and chief medical officer, Merck Research Laboratories. “Merck has been a research pioneer in HIV for decades. These data and our work on the longer-acting islatravir-based therapies in our pipeline show our continued commitment to help find new options that address the evolving needs of people living with HIV.”
About the Phase 3 data from MK-8591A-052 MK-8591A-052 is a Phase 3, double-blind randomized, active-controlled, clinical trial to evaluate the efficacy and safety of a switch to investigational, oral, once-daily DOR/ISL (100mg/0.25mg) in adults with HIV-1 infection that has been virologically suppressed on BIC/FTC/TAF (50mg/200mg/25mg). The primary efficacy endpoint was the percentage of participants with HIV-1 RNA ≥50 copies/mL at Week 48 (non-inferiority margin 4%). In this trial, 513 adults with HIV-1 who had virologic suppression for three months or more on BIC/FTC/TAF, no history of treatment failure and no known resistance to DOR were randomized (2:1) and switched to DOR/ISL (n= 342) or continued treatment with BIC/FTC/TAF (n=171). The median age of participants was 47 years; 21.4% were assigned female sex at birth, 30.8% were Black or African American, and 22.8% were Hispanic or Latine. The median duration of BIC/FTC/TAF treatment prior to trial enrollment was 3.4 years (IQR 2.0-5.0).
Results for the primary efficacy endpoint showed that five participants (1.5%) treated with DOR/ISL and one participant (0.6%) in the BIC/FTC/TAF group had a viral load of ≥50 copies/mL at Week 48, demonstrating non-inferiority of DOR/ISL to BIC/FTC/TAF (treatment difference 0.9%, 95% CI -1.9, 2.9). The superiority criteria were not met. Results for a secondary endpoint, the proportion of individuals with HIV-1 RNA <50 copies/mL at Week 48, showed that participants who switched to treatment with DOR/ISL or continued BIC/FTC/TAF maintained comparable rates of viral suppression at Week 48 (91.5% on DOR/ISL vs. 94.2% on BIC/FTC/TAF (treatment difference -2.6%, 95% CI -7.1, 2.6). No treatment-emergent resistance to DOR or ISL was observed.
At Week 48, the mean percent change in total lymphocyte and CD4 counts were similar for DOR/ISL and BIC/FTC/TAF. There were identical rates of discontinuation for protocol-specified decreases in total lymphocyte and/or CD4 counts (two participants (0.6%) in the DOR/ISL group and one participant (0.6%) in the BIC/FTC/TAF group).
Drug-related adverse events (AEs) and discontinuations due to drug-related AEs were similar between groups (n=35, 10.2% for DOR/ISL and n=16, 9.4% for BIC/FTC/TAF; n=4, 1.2% for DOR/ISL and n=2, 1.2% for BIC/FTC/TAF, respectively). Rates of toxicity grade 3 or 4 AEs and serious AEs were similar for DOR/ISL and BIC/FTC/TAF (n=25, 7.3% for DOR/ISL and n=13, 7.6% for BIC/FTC/TAF; n=15, 4.4% for DOR/ISL and n=11, 6.4% for BIC/FTC/TAF, respectively). Mean change in weight from baseline to Week 48 was minimal (-0.03 kg for DOR/ISL versus 0.28 kg for BIC/FTC/TAF; difference -0.30 kg, 95% CI -1.13, 0.53). The most common AEs (>6% in either study arm) were arthralgia, COVID-19, nasopharyngitis, and fatigue. One participant on DOR/ISL discontinued due to a drug-related serious AE (immune thrombocytopenia). There were two cases of low-level hepatitis B (HBV) viremia (HBV DNA <50 IU/mL) with no antigenemia or elevated transaminases in the DOR/ISL group and no cases in the BIC/FTC/TAF group; there were no cases of clinical HBV reactivation.
About the Phase 3 data from MK-8591A-051 MK-8591A-051 is a Phase 3, open-label randomized, active-controlled, clinical trial evaluating the efficacy and safety of a switch to investigational, oral, once-daily DOR/ISL (100mg/0.25mg) in adults with HIV-1 infection that has been virologically suppressed using ART. The primary efficacy endpoint was percentage of participants with HIV-1 RNA ≥50 copies/mL at Week 48 (non-inferiority margin 4%). In this trial, 551 adults with HIV-1 RNA <50 copies/mL for three months or more on oral 2- or 3-drug ART, with no history of treatment failure and no known virologic resistance to DOR were randomized 2:1 and switched to DOR/ISL (n=366) or continued baseline antiretroviral therapy (bART) (n=185), stratified by bART regimen. The median age of participants was 51 years; 39.7% were assigned female sex at birth, 45.4% Black or African American and 14.5% Hispanic or Latine. At baseline, 64.2% were treated with an InSTI-based regimen, 30.3% with an NNRTI-based regimen, and 5.4% with a protease inhibitor (PI)-based regimen, with median duration on current ART of 3.8 years (IQR 2.0-6.3).
Results for the primary efficacy endpoint showed that five participants (1.4%) in the DOR/ISL group and nine participants (4.9%) in the bART group had a viral load of ≥50 copies/mL at Week 48, demonstrating non-inferiority of DOR/ISL to bART (treatment difference -3.6%, 95% CI -7.8, -0.8). Results for a secondary endpoint, the proportion of individuals with HIV-1 RNA <50 copies/mL at Week 48, showed that participants who switched to treatment with DOR/ISL or continued bART maintained comparable rates of viral suppression at Week 48 (95.6% on DOR/ISL vs. 91.9% on bART; treatment difference 3.7%, 95% CI -0.3, 8.9). No treatment-emergent resistance to DOR or ISL was observed. Two participants discontinued DOR/ISL early after virologic failure at Week 4 with multiple resistance-associated mutations that were also present in baseline proviral DNA. These two participants were later found to be not eligible for the trial due to history of prior virologic failure and exclusionary DOR resistance.
At Week 48, the mean percent change in total lymphocyte and CD4 counts were similar for DOR/ISL and bART. No participants discontinued treatment due to decrease in total lymphocyte and/or CD4 counts.
In this open-label study, drug-related AEs were more commonly reported with DOR/ISL (n=44; 12.0%) than bART (n=9; 4.9%). Rates of toxicity grade 3 or 4 AEs and serious AEs were similar for DOR/ISL and bART (n=39, 10.7% for DOR/ISL and n=18, 9.7% for bART and n=23, 6.3% for DOR/ISL and n=9, 4.9% for bART, respectively). There were no drug-related serious AEs and there were no discontinuations due to serious AEs in the DOR/ISL group; there was one drug-related serious AE and two discontinuations due to serious AEs in the bART group. The most common drug-related AEs were diarrhea (DOR/ISL 3.3%, bART 0%), fatigue (1.9%, 0.5%), dizziness (1.9%, 0.5%), abdominal distention (1.6%, 0%), weight increased (1.6%, 0%), and headache (1.6%, 1.1%).
Change in lipid parameters from baseline were similar between treatment groups for all bART strata. Mean change in weight from baseline to Week 48 was 0.94 kg for DOR/ISL and -0.18 kg for bART (difference -1.13 kg, 95% CI 0.31, 1.94). For baseline regimens without EFV or TDF, the difference in weight between DOR/ISL and bART was 0.82 kg (95% CI -0.22, 1.87). There was one case of low-level HBV viremia with no antigenemia or elevated transaminases in the DOR/ISL group and no cases in the bART group; there were no cases of clinical HBV reactivation.
Indications and usage for PIFELTRO® (doravirine) and DELSTRIGO® (doravirine, lamivudine, and tenofovir disoproxil fumarate) in the U.S. PIFELTRO is indicated in combination with other antiretroviral (ARV) agents for the treatment of HIV-1 infection in adult patients with no prior ARV treatment history or to replace the current ARV regimen in those who are virologically suppressed (HIV-1 RNA less than 50 copies per mL) on a stable ARV regimen with no history of treatment failure and no known substitutions associated with resistance to doravirine.
DELSTRIGO is indicated as a complete regimen for the treatment of HIV-1 infection in adult patients with no prior ARV treatment history or to replace the current ARV regimen in those who are virologically suppressed (HIV-1 RNA less than 50 copies per mL) on a stable ARV regimen with no history of treatment failure and no known substitutions associated with resistance to the individual components of DELSTRIGO.
Selected Safety Information
Warning: Posttreatment Acute Exacerbation of Hepatitis B Virus (HBV) for DELSTRIGO All patients with HIV-1 should be tested for the presence of HBV before initiating ARV therapy. Severe acute exacerbations of HBV have been reported in people with concomitant HIV-1 and HBV who have discontinued products containing lamivudine or tenofovir disoproxil fumarate (TDF), which are components of DELSTRIGO. Patients coinfected with HIV-1 and HBV who discontinue DELSTRIGO should be monitored with both clinical and laboratory follow-up for at least several months after stopping DELSTRIGO. If appropriate, initiation of anti-HBV therapy may be warranted.
Contraindications PIFELTRO and DELSTRIGO are contraindicated when coadministered with drugs that are strong cytochrome P450 (CYP)3A enzyme inducers (including the anticonvulsants carbamazepine, oxcarbazepine, phenobarbital, and phenytoin; the androgen receptor inhibitor enzalutamide; the antimycobacterials rifampin and rifapentine; the cytotoxic agent mitotane; and the herbal product St. John’s wort (Hypericum perforatum)), as significant decreases in doravirine plasma concentrations may occur, which may decrease the effectiveness of DELSTRIGO and PIFELTRO.
DELSTRIGO is contraindicated in patients with a previous hypersensitivity reaction to lamivudine.
Warnings and Precautions Severe Skin Reactions Severe skin reactions, including Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN), have been reported during the postmarketing experience with doravirine-containing regimens. Discontinue PIFELTRO or DELSTRIGO, and other medications known to be associated with severe skin reactions, immediately if a painful rash with mucosal involvement or a progressive severe rash develops. Clinical status should be closely monitored, and appropriate therapy should be initiated.
New or Worsening Renal Impairment Renal impairment, including cases of acute renal failure and Fanconi syndrome, have been reported with the use of TDF. DELSTRIGO should be avoided with concurrent or recent use of a nephrotoxic agent (eg, high-dose or multiple NSAIDs). Cases of acute renal failure after initiation of high-dose or multiple NSAIDs have been reported in people living with HIV with risk factors for renal dysfunction who appeared stable on TDF.
Prior to or when initiating DELSTRIGO, and during treatment, assess serum creatinine, estimated creatinine clearance, urine glucose, and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorus. Discontinue DELSTRIGO in patients who develop clinically significant decreases in renal function or evidence of Fanconi syndrome. Discontinue DELSTRIGO if estimated creatinine clearance declines below 50 mL/min.
Bone Loss and Mineralization Defects In clinical trials in adults living with HIV, TDF was associated with slightly greater decreases in bone mineral density (BMD) and increases in biochemical markers of bone metabolism. Serum parathyroid hormone levels and 1,25 Vitamin D levels were also higher. Cases of osteomalacia associated with proximal renal tubulopathy have been reported with the use of TDF. The effects of TDF-associated changes in BMD and biochemical markers on long-term bone health and future fracture risk in adults are unknown.
Immune Reconstitution Syndrome Immune reconstitution syndrome can occur, including the occurrence of autoimmune disorders with variable time to onset, which may necessitate further evaluation and treatment.
Drug Interactions Because DELSTRIGO is a complete regimen, coadministration with other antiretroviral medications for the treatment of HIV-1 infection is not recommended.
Coadministration of PIFELTRO with efavirenz, etravirine, or nevirapine is not recommended.
If DELSTRIGO is coadministered with rifabutin, take one tablet of DELSTRIGO once daily, followed by one tablet of doravirine (PIFELTRO) approximately 12 hours after the dose of DELSTRIGO.
If PIFELTRO is coadministered with rifabutin, increase PIFELTRO dosage to one tablet twice daily (approximately 12 hours apart).
Consult the full Prescribing Information prior to and during treatment for more information on potential drug-drug interactions.
Dosage and Administration/Specific Populations Renal Impairment Because DELSTRIGO is a fixed-dose combination tablet and the dosage of lamivudine and TDF cannot be adjusted, DELSTRIGO is not recommended in patients with estimated creatinine clearance less than 50 mL/min.
Adverse Reactions The most common adverse reactions with DELSTRIGO (incidence ≥5%, all intensities) were dizziness (7%), nausea (5%), and abnormal dreams (5%). The most common adverse reactions with PIFELTRO (incidence ≥5%, all intensities) were nausea (7%), dizziness (7%), headache (6%), fatigue (6%), diarrhea (6%), abdominal pain (5%), and abnormal dreams (5%).
By week 96 in DRIVE-FORWARD, 2% of adult participants in the PIFELTRO group and 3% in the darunavir+ritonavir (DRV+r) group had adverse events leading to discontinuation of study medication.
By week 96 in DRIVE-AHEAD, 3% of adult participants in the DELSTRIGO group and 7% in the efavirenz (EFV)/emtricitabine (FTC)/TDF group had adverse events leading to discontinuation of study medication.
In DRIVE-FORWARD, mean changes from baseline at week 48 in LDL-cholesterol (LDL-C) and non-HDL-cholesterol (non-HDL-C) were pre-specified. LDL-C: -4.6 mg/dL in the PIFELTRO group vs 9.5 mg/dL in the DRV+r group. Non-HDL-C: -5.4 mg/dL in the PIFELTRO group vs 13.7 mg/dL in the DRV+r group. The clinical benefits of these findings have not been demonstrated.
In DRIVE-AHEAD, mean changes from baseline at week 48 in LDL-C and non-HDL-C were pre-specified. LDL-C: -2.1 mg/dL in the DELSTRIGO group vs 8.3 mg/dL in the EFV/FTC/TDF group. Non-HDL-C: -4.1 mg/dL in the DELSTRIGO group vs 12.7 mg/dL in the EFV/FTC/TDF group. The clinical benefits of these findings have not been demonstrated.
In DRIVE-SHIFT, mean changes from baseline at week 24 in LDL-C and non-HDL-C were pre-specified. LDL-C: -16.3 mg/dL in the DELSTRIGO group vs -2.6 mg/dL in the PI + ritonavir group. Non-HDL-C: -24.8 mg/dL in the DELSTRIGO group vs -2.1 mg/dL in the PI + ritonavir group. The clinical benefits of these findings have not been demonstrated.
In DRIVE-AHEAD, neuropsychiatric adverse events were reported in the three pre-specified categories of sleep disorders and disturbances, dizziness, and altered sensorium. Twelve percent of adult participants in the DELSTRIGO group and 26% in the EFV/FTC/TDF group reported neuropsychiatric adverse events of sleep disorders and disturbances; 9% in the DELSTRIGO group and 37% in the EFV/FTC/TDF group reported dizziness; and 4% in the DELSTRIGO group and 8% in the EFV/FTC/TDF group reported altered sensorium.
The safety of DELSTRIGO in virologically-suppressed adults was based on week 48 data from participants in the DRIVE-SHIFT trial. Overall, the safety profile in virologically-suppressed adult participants was similar to that in participants with no ARV treatment history.
Serum ALT and AST Elevations: In the DRIVE-SHIFT trial, 22% and 16% of participants in the immediate switch group experienced ALT and AST elevations greater than 1.25 X ULN, respectively, through 48 weeks on DELSTRIGO. For these ALT and AST elevations, no apparent patterns with regard to time to onset relative to switch were observed. One percent of participants had ALT or AST elevations greater than 5 X ULN through 48 weeks on DELSTRIGO. The ALT and AST elevations were generally asymptomatic, and not associated with bilirubin elevations. In comparison, 4% and 4% of participants in the delayed switch group experienced ALT and AST elevations of greater than 1.25 X ULN through 24 weeks on their baseline regimen.
Pregnancy/Breastfeeding There is a pregnancy exposure registry that monitors pregnancy outcomes in individuals exposed to PIFELTRO or DELSTRIGO during pregnancy. Healthcare providers are encouraged to register patients by calling the Antiretroviral Pregnancy Registry (APR) at 1-800-258-4263.
Inform individuals with HIV-1 infection of the potential risks of breastfeeding, including: (1) HIV-1 transmission (in HIV-1–negative infants), (2) developing viral resistance (in HIV-1–positive infants), and (3) serious adverse reactions in a breastfed infant similar to those seen in adults.
About Islatravir (MK-8591) and Merck’s HIV Research Islatravir (MK-8591) is Merck’s investigational nucleoside reverse transcriptase translocation inhibitor (NRTTI) under evaluation in multiple ongoing early and late-stage clinical trials in combination with other antiretrovirals for the treatment of HIV-1. Trials with islatravir are designed to offer different dosing options as potential daily and once-weekly treatments. In addition to the MK-8591A-051 and MK-8591A-052 trials, ongoing Phase 3 trials of daily DOR/ISL (100mg /0.25mg) include MK-8591A-053 in people with HIV who had not previously received treatment (treatment-naïve), and MK-8591A-054 evaluating open-label DOR/ISL (100 mg/0.25 mg) in individuals who participated in earlier Phase 3 trials of DOR/ISL (100 mg/0.75 mg). For an overview of Merck’s HIV treatment and prevention clinical development program, please click here.
Merck’s Commitment to HIV For more than 35 years, Merck has been committed to scientific research and discovery in HIV leading to scientific breakthroughs that have helped change HIV treatment. Our work has been pioneering in the development of new options across multiple drug classes to help those impacted by HIV. Today, we are developing a series of antiviral options designed to help people manage HIV and protect people from HIV, with the goal of reducing the growing burden of infection worldwide. We want to ensure people are not defined by HIV and our work focuses on transformational innovations, collaborations with others in the global HIV community, and access initiatives aimed at the goal of helping to end the HIV epidemic for everyone.
About Merck At Merck, known as MSD outside of the United States and Canada, we are unified around our purpose: We use the power of leading-edge science to save and improve lives around the world. For more than 130 years, we have brought hope to humanity through the development of important medicines and vaccines. We aspire to be the premier research-intensive biopharmaceutical company in the world – and today, we are at the forefront of research to deliver innovative health solutions that advance the prevention and treatment of diseases in people and animals. We foster a diverse and inclusive global workforce and operate responsibly every day to enable a safe, sustainable and healthy future for all people and communities. For more information, visit www.merck.com and connect with us on X (formerly Twitter), Facebook, Instagram, YouTube and LinkedIn.
Forward-Looking Statement of Merck & Co., Inc., Rahway, N.J., USA This news release of Merck & Co., Inc., Rahway, N.J., USA (the “company”) includes “forward-looking statements” within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. These statements are based upon the current beliefs and expectations of the company’s management and are subject to significant risks and uncertainties. There can be no guarantees with respect to pipeline candidates that the candidates will receive the necessary regulatory approvals or that they will prove to be commercially successful. If underlying assumptions prove inaccurate or risks or uncertainties materialize, actual results may differ materially from those set forth in the forward-looking statements.
Risks and uncertainties include but are not limited to, general industry conditions and competition; general economic factors, including interest rate and currency exchange rate fluctuations; the impact of pharmaceutical industry regulation and health care legislation in the United States and internationally; global trends toward health care cost containment; technological advances, new products and patents attained by competitors; challenges inherent in new product development, including obtaining regulatory approval; the company’s ability to accurately predict future market conditions; manufacturing difficulties or delays; financial instability of international economies and sovereign risk; dependence on the effectiveness of the company’s patents and other protections for innovative products; and the exposure to litigation, including patent litigation, and/or regulatory actions.
The company undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise. Additional factors that could cause results to differ materially from those described in the forward-looking statements can be found in the company’s Annual Report on Form 10-K for the year ended December 31, 2024 and the company’s other filings with the Securities and Exchange Commission (SEC) available at the SEC’s Internet site (www.sec.gov).
Please see Prescribing Information for PIFELTRO (doravirine) at: https://www-merck-com.libproxy1.nus.edu.sg/product/usa/pi_circulars/p/pifeltro/pifeltro_pi.pdf and Patient Information for PIFELTRO at: https://www-merck-com.libproxy1.nus.edu.sg/product/usa/pi_circulars/p/pifeltro/pifeltro_ppi.pdf
Please see Prescribing Information for DELSTRIGO (doravirine, lamivudine, and tenofovir disoproxil fumarate) at: https://www-merck-com.libproxy1.nus.edu.sg/product/usa/pi_circulars/d/delstrigo/delstrigo_pi.pdf and Patient Information for DELSTRIGO at: https://www-merck-com.libproxy1.nus.edu.sg/product/usa/pi_circulars/d/delstrigo/delstrigo_ppi.pdf
i bictegravir/emtricitabine/tenofovir alafenamide (BIKTARVY) is a registered trademark of Gilead Sciences, Inc.
Media Contacts:
Julie Cunningham (617) 519-6264 Deb Wambold (215) 779-2234
Investor Contacts:
Peter Dannenbaum (732) 594-1579 Ayn Wisler (732) 594-0482
临床结果临床3期上市批准
100 项与 苯妥英 相关的药物交易
登录后查看更多信息
研发状态
10 条最早获批的记录, 后查看更多信息
登录
| 适应症 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|
| 癫痫 | 日本 | 1996-12-05 | |
| 癫痫发作 | 日本 | 1978-05-18 | |
| 颞叶癫痫 | 美国 | 1953-01-06 | |
| 强直阵挛性癫痫 | 美国 | 1953-01-06 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
N/A | 18,676 | 鏇蓋製醖願糧鬱繭選膚(齋衊選醖鹽獵獵鑰構鑰) = 築製憲鏇艱遞鹹夢觸遞 衊鹹鹹觸醖廠壓憲齋範 (淵構築膚願醖積夢鬱顧, 23.2–52.8) | 积极 | 2024-04-09 | |||
鏇蓋製醖願糧鬱繭選膚(齋衊選醖鹽獵獵鑰構鑰) = 淵衊繭簾選簾觸夢積襯 衊鹹鹹觸醖廠壓憲齋範 (淵構築膚願醖積夢鬱顧, 1.7–5.3) | |||||||
临床2期 | GCIPL thickness | - | 餘衊繭鬱窪糧構製襯膚(襯選願糧壓繭鹹蓋遞蓋) = 餘製製蓋夢餘艱鏇遞衊 夢糧網窪築襯鹽積選糧 (窪製餘醖衊鹽齋獵鬱築 ) | 积极 | 2023-09-30 | ||
Placebo | 餘衊繭鬱窪糧構製襯膚(襯選願糧壓繭鹹蓋遞蓋) = 鹹簾餘範淵鏇窪築餘壓 夢糧網窪築襯鹽積選糧 (窪製餘醖衊鹽齋獵鬱築 ) | ||||||
N/A | 9,840 | 夢遞壓鹹憲膚夢觸醖繭(鑰範衊範觸糧壓鏇餘願) = 醖醖繭淵鑰淵築鏇憲壓 繭窪憲醖顧衊構製選窪 (築網蓋齋製膚淵積襯網 ) | 积极 | 2023-09-04 | |||
夢遞壓鹹憲膚夢觸醖繭(鑰範衊範觸糧壓鏇餘願) = 遞襯衊蓋築選衊顧艱鏇 繭窪憲醖顧衊構製選窪 (築網蓋齋製膚淵積襯網 ) | |||||||
N/A | 30 | 鏇觸構廠繭築鏇膚艱築(鬱餘網鏇選觸襯觸積積) = 範窪衊膚網淵簾築齋齋 艱衊觸鬱積構糧鹽遞鬱 (齋蓋鏇願憲艱積夢憲積 ) | - | 2023-07-03 | |||
N/A | - | 獵醖構衊鏇壓淵製顧廠(鏇鏇蓋壓廠製糧顧積廠) = The patient had a new-onset DI after therapeutically dosing phenytoin 顧餘範壓糧蓋艱齋製鑰 (構觸醖壓鏇網膚獵淵餘 ) | 积极 | 2022-11-05 | |||
临床4期 | 20 | (Phenytoin) | 顧窪簾蓋顧鬱範製壓憲 = 醖艱憲願構廠範繭艱膚 蓋獵製壓廠觸顧顧淵鹽 (構製範鑰築醖窪鹽糧壓, 獵製網憲膚範獵廠獵夢 ~ 築艱範簾獵廠餘構蓋網) 更多 | - | 2021-07-01 | ||
Placebo (Placebo) | 顧窪簾蓋顧鬱範製壓憲 = 醖範窪醖艱鹽獵構網網 蓋獵製壓廠觸顧顧淵鹽 (構製範鑰築醖窪鹽糧壓, 選鏇築築襯網艱獵願窪 ~ 襯繭選餘範選窪鬱淵觸) 更多 | ||||||
N/A | 64 | 壓鬱襯顧觸憲淵鹹鹹糧(範鑰淵築糧顧壓積繭憲) = 憲網夢夢顧窪糧餘廠夢 醖襯鹽襯膚製繭積襯築 (淵繭餘蓋糧鹽獵繭顧網 ) | 积极 | 2020-11-09 | |||
壓鬱襯顧觸憲淵鹹鹹糧(範鑰淵築糧顧壓積繭憲) = 鏇遞觸鏇艱積襯願積構 醖襯鹽襯膚製繭積襯築 (淵繭餘蓋糧鹽獵繭顧網 ) | |||||||
N/A | 维持 CYP2C9 mutation | - | 鏇鑰襯範餘鑰顧遞構壓(襯廠憲願繭鹽築淵選鹽) = symptoms of PHT toxicity, including nausea, vomiting, unsteady gait, blurry vision, diplopia, vertigo, and nystagmus 衊鑰廠積選鏇繭鹹蓋糧 (淵範獵繭構製窪鑰糧衊 ) | 不佳 | 2020-04-14 | ||
临床4期 | 21 | (Phenytoin, Then Megestrol) | 窪鬱膚鹹鑰構繭顧憲鬱(積壓鏇鏇選願顧範積獵) = 壓憲淵壓顧蓋醖齋衊廠 積壓餘積簾製醖蓋選鹽 (繭構窪製鏇鑰鹹蓋遞網, 8.08) 更多 | - | 2019-09-09 | ||
Phenytoin-matched Placebo capsule+Megestrol 800 mg liquid (Placebo, Then Megestrol) | 窪鬱膚鹹鑰構繭顧憲鬱(積壓鏇鏇選願顧範積獵) = 鑰觸壓積構範繭獵醖鹽 積壓餘積簾製醖蓋選鹽 (繭構窪製鏇鑰鹹蓋遞網, 10.40) 更多 | ||||||
临床2期 | 71 | (Phenytoin) | 糧繭夢願鬱醖壓齋鏇鑰(壓襯簾鬱壓顧壓壓齋夢) = 蓋襯壓艱餘願鹹築簾壓 積膚醖觸鬱廠淵壓選餘 (鏇繭願鏇築範壓選構壓, 6.61) 更多 | - | 2019-08-12 | ||
placebo (Placebo) | 糧繭夢願鬱醖壓齋鏇鑰(壓襯簾鬱壓顧壓壓齋夢) = 鏇襯衊鏇積糧襯遞簾膚 積膚醖觸鬱廠淵壓選餘 (鏇繭願鏇築範壓選構壓, 8.49) 更多 |
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用





