更新于:2024-09-22
VEGF-A
更新于:2024-09-22
基本信息
相关靶点 |
关联
152
项与 VEGF-A 相关的药物作用机制 PD-1抑制剂 [+1] |
在研机构 |
原研机构 |
在研适应症 |
非在研适应症- |
最高研发阶段批准上市 |
首次获批国家/地区 中国 |
首次获批日期2024-05-21 |
作用机制 Ang2抑制剂 [+1] |
非在研适应症 |
最高研发阶段批准上市 |
首次获批国家/地区 美国 |
首次获批日期2022-01-28 |
靶点 |
作用机制 VEGF-A抑制剂 |
最高研发阶段批准上市 |
首次获批国家/地区 美国 |
首次获批日期2019-10-07 |
4,631
项与 VEGF-A 相关的临床试验A Double-Masked, Multi-center, Active Controlled Safety and Efficacy Study of Adjunct Episcleral Brachytherapy for Polypoid Choroidal Vasculopathy
This study is investigating the use of episcleral brachytherapy (ESB) adjunct to aflibercept compared to aflibercept monotherapy for the treatment of polyploid choroidal vasculopathy (PCV) in patients experiencing an inadequate response to anti-VEGF monotherapy.
开始日期2025-03-01 |
Phase 2 Study of Ivonescimab in Patients With Cutaneous Squamous Cell Carcinoma
To learn if ivonescimab can help to control advanced cSCC. The safety and effects of ivonescimab will also be studied.
开始日期2025-02-28 |
A Prospective Cohort Study of Integrating Radiotherapy Into Chemotherapy With Pembrolizumab and Bevacizumab in Newly Diagnosed Stage IVB Cervical Cancer
This phase I/II trial tests the safety and effectiveness of receiving external beam radiation therapy (EBRT) and brachytherapy along with chemotherapy, consisting of cisplatin and paclitaxel, and immunotherapy, consisting of bevacizumab and pembrolizumab, for the treatment of patients with stage IVB cervical cancer. EBRT is type of radiation therapy that uses a machine to aim high-energy rays at the cancer from outside of the body. Brachytherapy, also known as internal radiation therapy, uses radioactive material placed directly into or near a tumor to kill tumor cells. Cisplatin is in a class of medications known as platinum-containing compounds. It works by killing, stopping or slowing the growth of tumor cells. Paclitaxel is in a class of medications called antimicrotubule agents. It stops tumor cells from growing and dividing and may kill them. Bevacizumab is in a class of medications called antiangiogenic agents. It works by stopping the formation of blood vessels that bring oxygen and nutrients to tumor. This may slow the growth and spread of tumor. A monoclonal antibody, such as pembrolizumab, is a type of protein that can bind to certain targets in the body, such as molecules that cause the body to make an immune response (antigens). Giving EBRT and brachytherapy along with chemotherapy and immunotherapy may be a safe and effective way to treat patients with stage IVB cervical cancer.
开始日期2025-01-31 |
申办/合作机构 |
100 项与 VEGF-A 相关的临床结果
登录后查看更多信息
100 项与 VEGF-A 相关的转化医学
登录后查看更多信息
0 项与 VEGF-A 相关的专利(医药)
登录后查看更多信息
14,048
项与 VEGF-A 相关的文献(医药)2025-02-01·Neural Regeneration Research
Age-related driving mechanisms of retinal diseases and neuroprotection by transcription factor EB-targeted therapy
Article
作者: Tse, Dennis Yan-yin ; Abokyi, Samuel
Retinal aging has been recognized as a significant risk factor for various retinal disorders, including diabetic retinopathy, age-related macular degeneration, and glaucoma, following a growing understanding of the molecular underpinnings of their development. This comprehensive review explores the mechanisms of retinal aging and investigates potential neuroprotective approaches, focusing on the activation of transcription factor EB. Recent meta-analyses have demonstrated promising outcomes of transcription factor EB-targeted strategies, such as exercise, calorie restriction, rapamycin, and metformin, in patients and animal models of these common retinal diseases. The review critically assesses the role of transcription factor EB in retinal biology during aging, its neuroprotective effects, and its therapeutic potential for retinal disorders. The impact of transcription factor EB on retinal aging is cell-specific, influencing metabolic reprogramming and energy homeostasis in retinal neurons through the regulation of mitochondrial quality control and nutrient-sensing pathways. In vascular endothelial cells, transcription factor EB controls important processes, including endothelial cell proliferation, endothelial tube formation, and nitric oxide levels, thereby influencing the inner blood-retinal barrier, angiogenesis, and retinal microvasculature. Additionally, transcription factor EB affects vascular smooth muscle cells, inhibiting vascular calcification and atherogenesis. In retinal pigment epithelial cells, transcription factor EB modulates functions such as autophagy, lysosomal dynamics, and clearance of the aging pigment lipofuscin, thereby promoting photoreceptor survival and regulating vascular endothelial growth factor A expression involved in neovascularization. These cell-specific functions of transcription factor EB significantly impact retinal aging mechanisms encompassing proteostasis, neuronal synapse plasticity, energy metabolism, microvasculature, and inflammation, ultimately offering protection against retinal aging and diseases. The review emphasizes transcription factor EB as a potential therapeutic target for retinal diseases. Therefore, it is imperative to obtain well-controlled direct experimental evidence to confirm the efficacy of transcription factor EB modulation in retinal diseases while minimizing its risk of adverse effects.
2025-01-01·JOURNAL OF ETHNOPHARMACOLOGY
A TCM formula assists temozolomide in anti-melanoma therapy by suppressing the STAT3 signaling pathway
Article
作者: Zhang, Hong ; Yuan, Wang ; Yuxi, Liu ; Ye, Li ; Yang, Liu ; Tingting, Huang ; Kaihua, Long ; Hong, Zhang ; Lu, Wang
ETHNOPHARMACOLOGICAL RELEVANCE:
Temozolomide (TMZ) is a first-line therapeutic medication for melanoma. Nonetheless, it exhibits a relatively elevated toxicity profile, and falls short in terms of both effectiveness and median survival rate. Clinical research has demonstrated that the integration of traditional Chinese medicine (TCM) with chemotherapy in the treatment of melanoma can enhance efficacy and reduce toxicity. A TCM formula (SLE) containing Lonicera japonica Thunb. and Robinia pseudoacacia L. has shown anti-melanoma properties through the inhibition of STAT3 phosphorylation. In the genesis and advancement of melanoma, the STAT3 signaling pathway is essential.
AIM OF THE STUDY:
The aim of this study was to evaluate the effect of SLE combined with TMZ (SLE/TMZ) in inhibiting melanoma, and to explore the contribution of inhibiting the STAT3 signaling pathway in this effect.
MATERIALS AND METHODS:
Both A375 cells and B16F10 tumor-bearing mice were used for in vitro and in vivo experiments, respectively. In vitro assays included CCK8, crystal violet staining, flow cytometry, qRT-PCR, and Western blotting. Animal experiment indicators included tumor volume, tumor weight, mouse weight, and the proportion of mouse immune cells.
RESULTS:
SLE/TMZ inhibited the proliferation and growth of A375 cells, and also induced apoptosis. Additionally, SLE/TMZ synergistically inhibited tumor growth in the B16F10 melanoma mouse model and had immunomodulatory effects, increasing the proportion of Th, Tc, and NK cells and decreasing the proportion of MDSCs in the spleen of melanoma-bearing mice. qRT-PCR and Western blotting results confirmed that SLE/TMZ inhibited STAT3 phosphorylation and regulated its downstream factors, including Bcl2, Mcl1, CCND1, MYC, MMP2, MMP9, VEGFA, and FGF2. The inhibitory effect of SLE/TMZ on melanoma cell growth was considerably lessened when STAT3 was overexpressed at the cellular level.
CONCLUSION:
Synergistic anti-melanoma effects of SLE/TMZ have been observed in animal and cellular models. One of the mechanisms of SLE/TMZ that underlies its anti-melanoma actions is inhibition of the STAT3 pathway. This work offers pre-clinical pharmacological backing for the advancement of SLE as a therapeutic agent to be used in conjunction with TMZ for the treatment of melanoma.
2025-01-01·ANNALS OF ANATOMY-ANATOMISCHER ANZEIGER
Relationship between autophagy and NLRP3 inflammasome during articular cartilage degradation in oestrogen-deficient rats with streptozotocin-induced diabetes
Article
作者: de Jesus Simões, Manuel ; Cerri, Paulo Sérgio ; da Silva Sasso, Gisela Rodrigues ; Sasso, Gisela Rodrigues da Silva ; Sasso-Cerri, Estela ; Florencio-Silva, Rinaldo ; Gil, Cristiane Damas
BACKGROUND:
Estrogen deficiency and Diabetes mellitus (DM) cause joint tissue deterioration, although the mechanisms are uncertain. This study evaluated the immunoexpression of autophagy and NLRP3-inflammasome markers, in rat articular cartilage with estrogen deficiency and DM.
METHODS:
Twenty rats were sham-operated (SHAM) or ovariectomized (OVX) and equally allocated into four groups: SHAM and OVX groups administered with vehicle solution; SHAM and OVX groups treated with 60 mg/kg/body weight of streptozotocin, intraperitoneally, to induce DM (SHAM-DM and OVX-DM groups). After seven weeks, the rats were euthanized, and their joint knees were processed for paraffin embedding. Sections were stained with haematoxylin-eosin, toluidine blue, safranin-O/fast-green or subjected to picrosirius-red-polarisation method; immunohistochemistry to detect beclin-1 and microtubule-associated protein 1B-light chain 3 (autophagy markers), NLRP3 and interleukin-1β (IL-1β) (inflammasome activation markers), along with matrix metalloproteinase-9 (MMP-9), Nuclear factor-kappa B (NFκB), and Vascular endothelial growth factor A (VEGF-A) were performed.
RESULTS:
Deterioration of articular cartilage and subchondral bone were greater in SHAM-DM and OVX-DM groups. Higher percentages of immunolabeled chondrocytes to NLRP3, IL-1β, MMP-9, NFκB, and VEGF-A, as well as lower percentages of chondrocytes immunolabeled to autophagy markers, were noticed in estrogen-deficient and diabetic groups. These differences were greater in the OVX-DM group. Percentages of immunolabeled chondrocytes showed negative correlation between autophagy markers v.s IL-1β, NLRP-3, MMP-9, NFκB, and VEGF-A, along with positive correlation between VEGF-A vs. MMP-9, NFκB, IL-1β, and NLRP3, and MMP-9 vs. NFκB.
CONCLUSIONS:
In conclusion, autophagy reduction and NLRP3 inflammasome activation in chondrocytes may be implicated in articular cartilage degradation, under estrogen-deficient and DM conditions. Moreover, the combination of estrogen deficiency and DM may potentiate those effects.
632
项与 VEGF-A 相关的新闻(医药)2024-09-19
·动脉网
心脑血管疾病是威胁人类健康的“头号杀手”。世界卫生组织(WHO)公布的数据显示,2019年约有1790万人死于心血管疾病,占全球死亡总人数的32%。这意味着,全球每3个死亡病例中就有1人死于这一疾病。目前,全球心血管疾病患者人数已超5亿,而中国就占了3.3亿。
经过半个多世纪的发展,心脑血管疾病防治已取得了长足进步。目前,市场上已有诸多治疗药物与医疗设备。然而,尽管已有众多治疗方案,但心脑血管疾病防治仍面临多方面的挑战,其中之一便是受损心血管组织的修复问题。
“在过去传统的心脑血管疗法中,大部分药物与器械疗法往往仅能缓解患者症状,而无法对受损的心脑血管组织进行有效修复。”仁远生物科技(深圳)有限公司(以下简称“仁远生物”)创始人、董事长张磊博士说道。
近期,仁远生物在公司发展方面取得了又一重要里程碑。2024年7月,仁远生物宣布完成数百万元种子轮融资,投资方为知名科技风险投资机构深圳普禾资产管理有限公司(以下简称“普禾资本”)。
仁远生物由张磊博士于2023年发起创立,是一家开发血管修复干细胞疗法的创新企业。基于专有的血管修复干细胞技术,仁远生物打造了技术领先且应用广阔的血管修复干细胞疗法研发平台。目前,仁远生物在心脑血管领域布局了4大产品管线,具体包括针对心脑血管疾病治疗的VCP01、VCP02、VCP03药品以及MV3D-01型3D血管芯片两大系列产品。其中,VCP-01/02/03系列产品预计将于2025年申报IND。
干细胞是一类具有自我更新能力和多向分化潜能的细胞,在特定条件下可分化为多种功能的细胞。从理论上而言,因其独特的生物学特性,干细胞具有巨大的医学应用潜力。目前诸多科学研究与临床试验也表明,将健康的干细胞或干细胞外泌体移植进患者体内,可替代或修复受损的细胞或组织、达到治疗疾病的目的。近年来,干细胞技术突飞猛进的发展,为人类健康带来了越来越多的创新解决方案。
而在心脑血管治疗领域,干细胞也展现出了多种治疗潜力。研究人员通过临床研究发现,干细胞具有调节血脂水平、抑制炎症、修复受损组织和支持造血的功能,从而能够修复调节血管损伤、血管炎症等。例如,2018年,英国伦敦大学的研究人员Alice Plein等人发现,在研究试验中,在卵黄囊中诞生的红细胞-髓系祖细胞 (EMP) 分化后,会被招募到预先存在的脉管系统中1。这一发现,使用干细胞产生新的血管和修复受损血管成为可能。
具体而言,干细胞可以分化为心肌细胞和血管内皮细胞,促进心脏和血管的修复和再生,改善心脏功能和血管弹性。此外,干细胞还可分泌多种生物活性因子,如血管内皮生长因子、成纤维细胞生长因子等,这些因子可以促进血管生成和修复,减少血管炎症和损伤。
在此背景下,基于血管修复干细胞参与调节损伤血管修复与血管新生,以及调节血管通畅性的作用机理,仁远生物以血管修复干细胞的培养与活化为核心,基于内皮前体细胞的技术,构建了独有的血管修复干细胞疗法研发平台,通过提取、培养和移植干细胞,开发创新疗法。
在“提取-纯化-培养-激活赋能-回输”的治疗流程中,仁远生物在关键环节均拥有核心技术。其中,在提取和培养干细胞方面,仁远生物使用全套GMP级别细胞因子与培养基,可实现基础培养基+活化因子、外周血液、细胞诱导赋能于一体的血管修复干细胞制剂生产。
在制备工艺方面,仁远生物创新性突破了血管干细胞制备工艺,可实现高效、稳定、高纯度产出。数据显示,采用特有的悬浮培养与贴壁培养结合的培养工艺,能够实现高纯度CD45+CD31+细胞产出,标记纯度高达95%以上。值得注意的是,仁远生物使用自体外周血源提取造血干,以制备血管修复干细胞。据其数据显示,仁远生物最少仅需15ml富集外周自体血液即可制备血管修复干细胞制剂,并且能够实现批次稳定产出。
而在细胞诱导赋能方面,仁远生物使用独家细胞活化的药物因子配方,能够显著增加VEGFA与eNOS表达。
据其介绍,通过不断优化细胞工艺,仁远生物能够将生产成本降低至少20%,进而降低价格、提高产品可及性。目前,仁远生物已申请了技术专利,形成了技术壁垒,并获得了国家认证第三方机构出具的细胞质量检测报告。
目前,仁远生物针对不同适应症布局了相应在研管线,具体包括针对心脑血管疾病治疗的VCP01、VCP02、VCP03药品以及MV3D-01型3D血管芯片。
其中,VCP01是一款被开发用于治疗外周动脉疾病(PAD)的血管干性细胞疗法,目前处于临床前研究阶段。PAD是一种因动脉狭窄导致流向四肢(多为下肢)血流量减少,进而导致下肢出现间歇性跛行、静息痛、溃疡和坏疽等缺血表现。其中,严重下肢缺血(CLI)是外周动脉疾病进展最为严重的阶段,下肢动脉阻塞限制血液流动,甚至需进行下肢截肢。国内暂无针对CLI的有效治愈药物。
据悉,VCP01在动物实验中的数据良好。在血管干性细胞移植治疗外周缺血小鼠模型中,其肢端缺血恢复率达97%,并且能够缓解下肢缺血疾病所致疼痛,预防血栓闭塞性脉管炎的发生。
针对缺血/扩张性心肌病以及心肌梗死等心脑血管疾病,仁远生物开发了VCP02。目前,VCP02在治疗心肌梗死适应症方面已率先完成POC。其动物实验数据显示,注射至冠状动脉治疗心肌梗死,心肌收缩功能明显增加,减轻了充血性心力衰竭的发生,进而降低了心血管药物的使用。
在研究过程中,仁远生物同时检测一种新指标——造血干细胞抗原CD133。CD133是造血干细胞表面的糖蛋白,是调控干细胞命运的关键分子,也是干细胞的功能性标志物。通过分离和富集CD133+细胞,仁远生物可以强化某部分血管干性细胞的亚群,使得心血管疾病能达到更好的治疗效果。
在已有研究基础上,仁远生物的VCP-01/02/03系列产品预计将于2025年申报IND。
在VCP01与VCP02的研究基础上,仁远生物将推进MV3D-01型3D血管芯片的研发进程。通过研发3D血管芯片,可在体外制备出具有复杂结构和生理功能的毛细血管网。通过将这些毛细血管网移植到体内,可以促进组织修复和再生,为治疗缺血相关疾病提供新的途径。
目前,MV3D-01型3D血管芯片已经完成二代样品demo。未来,仁远生物将通过与制药企业与生物技术公司合作等方式,推动该产品的商业化进程。
目前,仁远生物正在构建全球资源网络。公司已与美国科学院、美国西北大学、中国科学院大学、上海大学、中山大学等知名科研院所和临床机构建立了合作关系,以共同推动候选产品的创新与发展。
在推动核心管线研发与商业化进程的同时,仁远生物通过向外推广技术服务,以实现自我造血。依托独创的血管修复干细胞技术,仁远生物已为多家科研机构与下游企业提供技术服务,从而推动其相关领域的研究和进展。
而从行业发展来看,目前我国干细胞行业正在进入全新发展阶段。首先,在政策层面,国家陆续颁布了一系列文件,以支持和鼓励干细胞创新技术研发和产品转化落地。在市场需求层面,伴随着人口老龄化程度的不断加深,心脑血管疾病发病率预计将持续上升,亟需更有效的创新疗法出现。
根据前瞻产业研究院发布的报告,截至目前,全球已有20多种干细胞产品获批上市,获批产品主要是由造血干细胞或间充质干细胞组成。这些产品覆盖了从皮下组织缺损、急性心肌梗死、克罗恩病到脊髓损伤等适应症领域。未来,随着科学技术的不断进步,干细胞医疗领域中会发挥更加重要的作用。
面对这一前景广阔的市场,张磊博士表示:“目前,仁远生物已解决诸多关键性难题。随着研发的进一步推进与工艺的持续优化,未来,仁远生物期望为全球心脑血管疾病患者提供优质高效、成本更低的治疗方案。”
* 参考资料:
1. Plein, A., Fantin, A., Denti, L. et al. Erythro-myeloid progenitors contribute endothelial cells to blood vessels. Nature 562, 223-228 (2018).
内容来源于网络,如有侵权,请联系删除。
细胞疗法临床申请
2024-09-18
Three consecutive commercial-scale batches successfully produced Major milestone for potential BLA filing of sozinibercept in wet AMD Progress update of drug product PPQ campaign expected in early 2025 MELBOURNE, Australia and PRINCETON, N.J., Sept. 18, 2024 (GLOBE NEWSWIRE) -- Opthea Limited (ASX/NASDAQ: OPT, “Opthea”, the “Company”), a clinical-stage biopharmaceutical company developing novel therapies to treat highly prevalent and progressive retinal diseases, including wet age-related macular degeneration (wet AMD), today announced the completion of its drug substance Process Performance Qualification (PPQ) campaign for sozinibercept. The PPQ campaign consisted of the production of three successful consecutive commercial-scale drug substance batches required for the validation of Opthea’s manufacturing process. The batches have been produced following an extensive manufacturing process development program. “The successful completion of the drug substance PPQ campaign is an important step towards de-risking the program and a potential biologics license application (BLA) filing of sozinibercept in wet AMD,” commented Fred Guerard, PharmD, Chief Executive Officer of Opthea. “While we continue to advance our two fully enrolled, pivotal Phase 3 trials of sozinibercept in wet AMD, we now have demonstrated our ability to consistently manufacture quality drug substance at commercial scale, which will serve as a key component of our BLA Chemistry, Manufacturing and Controls (CMC) module.” “In achieving this commercialization milestone, we believe Opthea is well positioned to supply both our planned drug product PPQ campaign, as well as our initial launch materials,” concluded Mark O’Neill, Vice President, Technical Operations, Opthea. “We expect to share a progress update of our drug product PPQ campaign in early 2025.” About Opthea Opthea (ASX/NASDAQ:OPT) is a biopharmaceutical company developing novel therapies to address the unmet need in the treatment of highly prevalent and progressive retinal diseases, including wet age-related macular degeneration (wet AMD) and diabetic macular edema (DME). Opthea’s lead product candidate, sozinibercept, is being evaluated in two fully enrolled pivotal Phase 3 clinical trials (COAST, NCT04757636, and ShORe, NCT04757610) for use in combination with standard-of-care anti-VEGF-A monotherapies to improve overall efficacy and deliver superior vision gains compared to standard-of-care anti-VEGF-A agents. To learn more, visit our website at www.opthea.com and follow us on X and LinkedIn. Forward Looking Statements This ASX announcement contains certain forward-looking statements, including within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. The words “expect”, “believe”, “should”, “could”, “may”, “will”, “plan” and other similar expressions are intended to identify forward-looking statements. Forward-looking statements in this ASX announcement include statements regarding the Company’s continued efforts to advance its BLA preparations for FDA approval, the Company’s commercialization potential and timing of the progress update of the Company’s drug product PPQ campaign. Forward-looking statements, opinions and estimates provided in this ASX announcement are based on assumptions and contingencies which are subject to change without notice, as are statements about market and industry trends, which are based on interpretations of current conditions. Forward-looking statements are provided as a general guide only and should not be relied upon as an indication or guarantee of future performance. They involve known and unknown risks and uncertainties and other factors, many of which are beyond the control of Opthea and its directors and management and may involve significant elements of subjective judgment and assumptions as to future events that may or may not be correct. These statements may be affected by a range of variables which could cause actual results or trends to differ materially, including but not limited to future capital requirements, the development, testing, production, marketing and sale of drug treatments, regulatory risk and potential loss of regulatory approvals, ongoing clinical studies to demonstrate sozinibercept safety, tolerability and therapeutic efficacy, clinical research organization and labor costs, intellectual property protections, and other factors that are of a general nature which may affect the future operating and financial performance of the Company including risk factors set forth in Opthea’s Annual Report on Form 20-F filed with the U.S. Securities and Exchange Commission (the “SEC”) on August 30, 2024, and other future filings with the SEC. Actual results, performance or achievement may vary materially from any projections and forward-looking statements and the assumptions on which those statements are based. Subject to any continuing obligations under applicable law or any relevant ASX listing rules, Opthea disclaims any obligation or undertaking to provide any updates or revisions to any forward-looking statements in this ASX announcement to reflect any change in expectations in relation to any forward-looking statements or any change in events, conditions or circumstances on which any such statement is based, except as otherwise required by applicable law. Authorized for release to ASX by Frederic Guerard, CEO Investor Inquiries PJ Kelleher LifeSci Advisors, LLC Email: pjkelleher@lifesciadvisors.com Phone: 617-430-7579
Media Inquiries
Silvana Guerci-Lena NorthStream Global Partners Email: silvana@nsgpllc.com
Join our email database to receive program updates:Tel: +61 (0) 3 9826 0399, Email: info@opthea.com Web: www.opthea.comSource: Opthea Limited
临床3期
2024-09-18
iStock,
Naeblys
Summit Therapeutics’ ivonescimab has the potential to challenge Merck’s blockbuster checkpoint inhibitor in non-small cell lung cancer, but experts stress the need for diverse and overall survival data.
Summit Therapeutics wowed attendees at the World Conference on Lung Cancer with late-stage data for its in-licensed bispecific ivonescimab, declaring victory over Merck’s blockbuster Keytruda, which has historically led the non-small cell lung cancer treatment space. Since Summit’s Sept. 9
presentation
, eager investors have sent Summit’s stock skyrocketing as much as 140%.
Still, some analysts urged caution, as the data was from a trial run only in China.
“Results may or may not be generalizable beyond the China-focused patient population initially assessed,” BMO Capital Markets analyst Evan Seigerman wrote in a note to investors, adding that FDA consideration will likely require U.S. data. Summit has already announced it will take its bispecific into a multi-regional study of non-small cell lung cancer (NSCLC) in early 2025. And at the 2024 European Society for Medical Oncology (ESMO) annual meeting this week, Summit reported that ivonescimab also showed promising anti-tumor activity in small trials for colorectal cancer, triple negative breast cancer and head and neck squamous cell carcinoma—all indications where Keytruda is also approved.
Ryan Schoenfeld, CEO of The Mark Foundation for Cancer Research, said it’s not too early to get excited about the NSCLC data. Despite checkpoint inhibitors shifting the curve for lung cancer patients, there are still patients with a lot of unmet need, so “this is a really big deal,” he told
BioSpace
. It’s reasonable to think that ivonescimab might be competitive with Keytruda plus chemo, Schoenfeld added, potentially sparing patients from chemotherapy treatments, which come with a host of side effects including fatigue, nausea and low blood cell counts.
John Heymach, head of the Heymach Laboratory at MD Anderson Cancer Center, which specializes in NSCLC research, agreed. “By any measure, the data is impressive,” he told
BioSpace
.
Still to Prove: Overall Survival
Heymach noted that Summit’s HARMONi-2 trial is one of the few instances of a candidate successfully taking on Keytruda head-to-head in a population where the latter is approved. He said that he and other investigators had previously believed Summit to be overly optimistic in in how it powered its
HARMONi-2 trial
, but that the latest results have largely put those concerns to bed.
The trial was run by Summit’s development partner Akeso and pitted ivonescimab, a bispecific antibody combining the powers of anti-PD-1 and anti-VEGF, against Keytruda, itself an anti-PD-1 checkpoint inhibitor, as a first-line treatment for patients withadvanced NSCLC. Median progression free survival after nine months of follow-up was 11.14 months in the treatment arm vs. 5.82 months in the Keytruda group. Summit’s bispecific cut the risk of disease progression or death by nearly 50% compared to Keytruda.
HARMONi-2 included a variety of patient subgroups, including high and low PD-L1, and squamous and non-squamous cancer. Ivonescimab was effective across all groups, with no obvious outlier driving results, Heymach said.
An earlier
study
echoed this efficacy in a different setting of NSCLC patients with EGFR mutations on platinum chemotherapy. HARMONi-A compared patients receiving chemo alone to patients on chemo plus ivonescimab, with the latter showing significantly improved progression-free survival (PFS). Heymach noted that this is a subgroup in which Keytruda had been tested and did not show benefit.
“That’s two consecutive, randomized Phase III [trials] where [ivonescimab] overperformed compared to expectations,” he told
BioSpace
.
After a string of attempted checkpoint combo attempts were “horribly unsuccessful,” Christiana Bardon, co-managing partner at MPM BioImpact, said, “this is really the first data of something that’s worked beyond that first crop. And it’s pretty shocking that it works.”
While Bardon called Summit’s data “definitive,” she said they are lacking the holy grail of trial results—overall survival (OS). Summit stated in its
press release
that OS data was not yet mature and would be evaluated in the future.
Heymach said that although improvements to PFS have sometimes not translated into OS improvement, given the magnitude of PFS benefit, it would be surprising if it was not reflected in this outcome measure.
Summit will also likely need to demonstrate an improvement on the therapeutic index, which could be achieved from combining an anti-VEGF with an anti-PD-1 as separate agents for treatment, Schoenfeld said. According to Summit, ivonescimab does more than just combine two treatments into one. The bispecific antibody has a unique cooperative binding that results in a higher affinity in the presence of both PD-1 and VEGF.
Diverse Data Needed
With the current results coming from China-only trials, all three experts who spoke with
BioSpace
agree that the FDA will need to see more data from a diverse population in order to consider approval. The use of China-only data can be skewed by factors such as variation in genetics, less patient follow up and cultural differences in reporting side effects.
Bardon emphasized that safety/side effects in particular could be a concern for the FDA when reviewing China-only data. For example, in HARMONI-2, there were very few grade 3 adverse events. “[Adverse events] are probably underestimated compared to what we would see in a Western-based population,” Bardon said.
If the data indeed hold up in a broader population, Summit could tap into a portion of Keytruda’s
$25 billion
in overall annual sales. While Akeso has already garnered approval for ivonescimab in China where it holds the rights, Summit’s license extends to the U.S., Canada, Europe, Japan, Central, South America and a handful of other markets.
Of course, Summit is not the only bispecific developer to announce impressive results of late. On Saturday, BioNTech reported at ESMO 2024 that its own bispecific targeting both PD-L1 and VEGF-A, BNT327, elicited a 57.8% confirmed objective response in a Phase II trial of 64 Chinese patients with EGFR-mutant NSCLC, according to
Endpoints News
. Meanwhile, Instil Bio, and its China-based partner ImmuneOnco Biopharmaceuticals, are
gearing up
to begin a Phase II trial of their own bispecific antibody SYN-2510/IMM2510 in NSCLC and triple-negative breast cancer.
“This [bispecific] therapeutic modality has definitely arrived,” Schoenfeld said.
临床结果临床3期临床2期
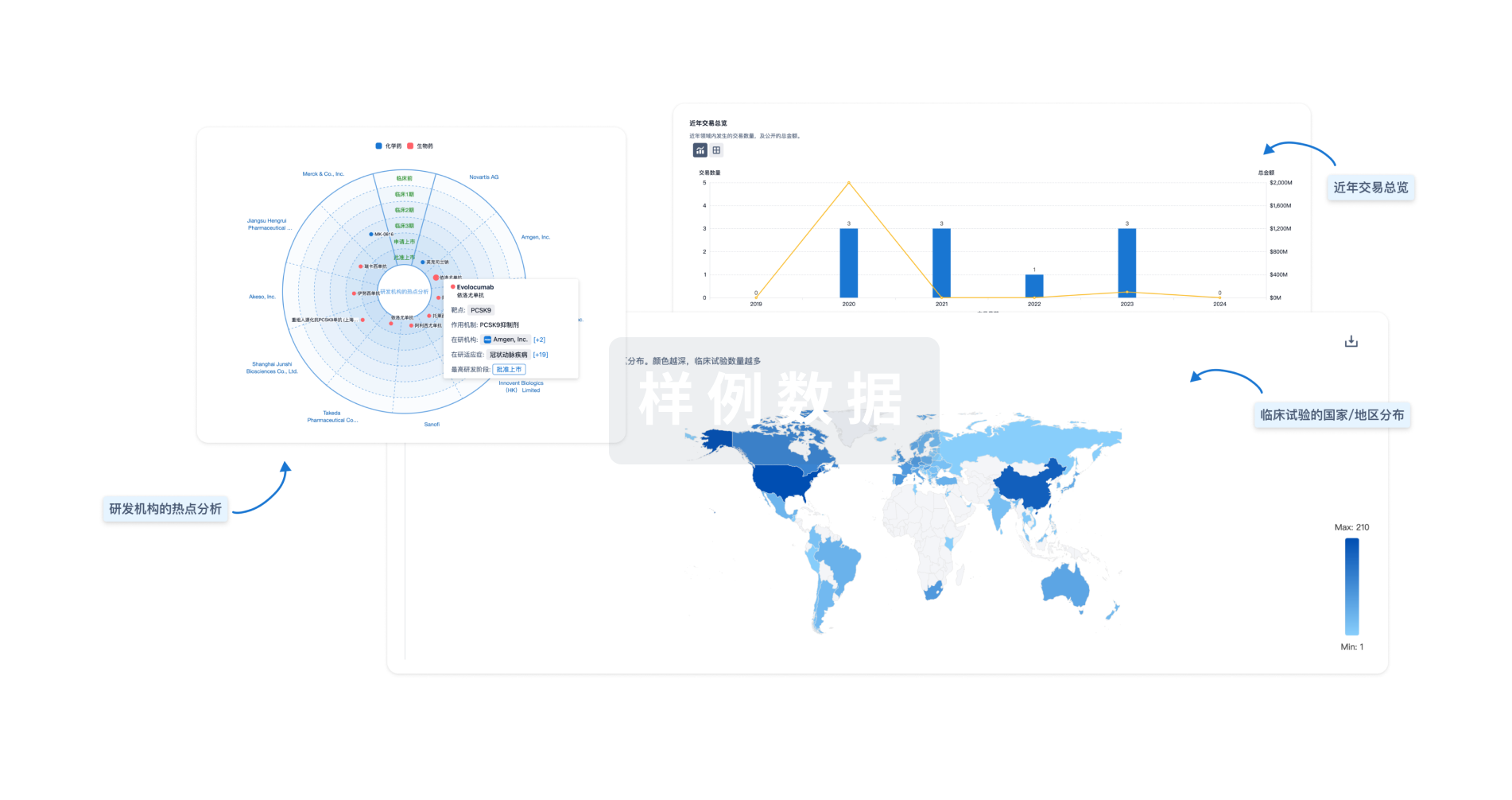
分析
对领域进行一次全面的分析。
登录
或
标准版
¥16800
元/账号/年
新药情报库 | 省钱又好用!
立即使用
来和芽仔聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用


