更新于:2024-11-01

Jiangsu Nhwa Pharmaceutical Co., Ltd.
更新于:2024-11-01
概览
标签
其他疾病
神经系统疾病
感染
小分子化药
化学药
疫苗
疾病领域得分
一眼洞穿机构专注的疾病领域
暂无数据
技术平台
公司药物应用最多的技术
暂无数据
靶点
公司最常开发的靶点
暂无数据
| 排名前五的药物类型 | 数量 |
|---|---|
| 小分子化药 | 38 |
| 化学药 | 5 |
| 疫苗 | 1 |
| 重组多肽 | 1 |
关联
55
项与 江苏恩华药业股份有限公司 相关的药物作用机制 μ opioid receptor激动剂 |
在研机构 |
原研机构 |
在研适应症 |
最高研发阶段批准上市 |
首次获批国家/地区 美国 |
首次获批日期2020-08-07 |
作用机制 COX-1抑制剂 [+1] |
在研机构 |
原研机构 |
最高研发阶段批准上市 |
首次获批国家/地区 中国 |
首次获批日期2006-10-27 |
112
项与 江苏恩华药业股份有限公司 相关的临床试验一项评价NH600001乳状注射液在健康受试者中持续输注的安全性、耐受性及药代/药效动力学的单中心、随机、双盲、丙泊酚阳性对照的I期临床研究
主要目的:评价NH600001乳状注射液在健康受试者中持续输注的安全性和耐受性。
次要目的:评价NH600001乳状注射液在健康受试者中持续输注的PK和PD特征。
开始日期2024-08-20 |
申办/合作机构 |
枸橼酸芬太尼口腔黏膜贴片的人体生物等效性研究(预试验)
以江苏恩华药业股份有限公司研制、生产的枸橼酸芬太尼口腔黏膜贴片(0.4 mg/片)为受试制剂,TEVA PHARMACEUTICALS EUROPE B.V.生产的枸橼酸芬太尼口腔黏膜贴片(0.4 mg/片,商品名:EFFENTORA ®)为参比制剂,研究受试制剂和参比制剂在中国轻度慢性疼痛志愿者空腹条件下单剂量给药时的药代动力学特征,并初步评价两制剂空腹条件下的人体生物等效性,为后期正式生物等效性试验方案的采血点设计等提供依据
开始日期2024-08-10 |
申办/合作机构 |
一项评价NH600001乳状注射液在健康非老年与老年受试者中单次给药的药代动力学临床试验
主要目的: 评价健康非老年与老年受试者单次静脉注射NH600001乳状注射液的药代动力学特征,为NH600001乳状注射液的老年人用药提供指导。 次要目的:
评价健康非老年与老年受试者单次静脉注射NH600001乳状注射液的药效动力学和安全性。
开始日期2024-08-07 |
申办/合作机构 |
100 项与 江苏恩华药业股份有限公司 相关的临床结果
登录后查看更多信息
0 项与 江苏恩华药业股份有限公司 相关的专利(医药)
登录后查看更多信息
40
项与 江苏恩华药业股份有限公司 相关的文献(医药)2024-11-01·Brain Research
Inhibiting NF-κB inducing kinase improved the motor performance of ALS animal model
Article
作者: Guo, Moran ; Yi, Le ; Liu, Yakun ; Duan, Weisong ; Li, Chunyan ; Li, Zhongyao ; Cao, Mengjie ; Li, Yuanyuan ; Tian, Yunyun ; Bi, Yue ; Xu, Xiangyang ; Sun, Jiaquan ; Xu, Yuyan
2023-11-01·Acta Pharmaceutica Sinica B
Carbazole and tetrahydro-carboline derivatives as dopamine D3 receptor antagonists with the multiple antipsychotic-like properties
Article
作者: Jiao, Peili ; Qiu, Yinli ; Li, Yiyan ; Zheng, Ruqiu ; Zhu, Guiwang ; Fang, Fan ; Zhang, Guisen ; Lv, Xuehui ; Wang, Yuxi ; Zhang, Liangren ; Li, Zhongtang ; Li, Zhongjun ; Jin, Zefang ; Liu, Zhenming ; Xu, Xiangqing
2023-03-01·European Journal of Medicinal Chemistry
2,6-diazaspiro[3.4]octan-7-one derivatives as potent sigma-1 receptor antagonists that enhanced the antinociceptive effect of morphine and rescued morphine tolerance
Article
作者: Fu, Kequan ; Xiong, Bing ; Zhao, Huimin ; Qiu, Yinli ; Guo, Dong ; Chen, Danqi ; Yang, Ruicong ; Xu, Huanyu ; Wei, Yaqin ; Liu, Hongli ; Xu, Wen
241
项与 江苏恩华药业股份有限公司 相关的新闻(医药)2024-10-30
·梯瓦医药
以下内容转自“澎湃新闻”。
“从2018年到现在,在六年的时间里,我们已经有6种品牌药以及1款健康消费品进入中国市场。实实在在看到了中国日益多元的市场需求。”第七届中国国际进口博览会即将开幕,梯瓦大中华区总经理黄迪仁在接受采访时表示,梯瓦将继续深化“在中国,为中国”战略,希望能把更多的优质医药产品带给中国患者。
近120多年历史的梯瓦,是全球领先的生物医药企业,因其在仿制药领域上的强劲实力,而被外界熟知。发展至今,梯瓦拥有全面多元的产品线,覆盖仿制药、创新药、生物类似药、非处方药等领域。2018年,梯瓦进入中国,用百年历史积淀形成的多元化产品线,为中国市场提供着覆盖中枢神经系统、肿瘤、皮肤管理、抗感染等领域产品资源。
黄迪仁提到,梯瓦受益于进博会的平台优势,已然成为中国市场的深耕者。今年进博会,梯瓦将带来两款中国“首展”、“首秀”的产品,并将进一步深化和拓展与本土伙伴的合作,携手推动医疗行业的高质量发展。
01
赋能健康未来
进博老友再上新
第七届进博会,梯瓦以全新主题“全力以赴,共筑健康未来”参展。
黄迪仁表示,“‘全力以赴,共筑健康未来’是梯瓦在去年发布的全新企业使命,代表着梯瓦创立120多年来的不变初心,和对于改善各国人民健康的坚定决心。以全新主题参展,不仅是对梯瓦企业使命的重申,也展现了我们对中国市场的长期承诺,以及全面助力健康中国2030实现的决心。”
对于很多跨国药企来说,进博会可以让大众了解到他们的最新药品以及能为中国患者提供的价值。在过去两年的进博旅程中,梯瓦不断展示着医药产品方面的深厚积淀,多款产品受益于不断释放的溢出效应,更快、更深入地为中国患者带来福音。
其中,创新药安泰坦®(氘丁苯那嗪片)是一个生动的例子。作为中国首个获批的氘代药物,安泰坦®在治疗成人与亨廷顿病有关的舞蹈病(Huntington's Disease, HD)及迟发性运动障碍(Tardive Dyskinesia, TD)方面表现出色,填补了相关治疗领域的空白。2020年,它被纳入医保目录,提升患者治疗的可负担性。
在2022年的进博会上,安泰坦®和罕见病亨廷顿病都受到了更广泛的关注。而在2023年,安泰坦®更是成功续约医保目录,长期降低了患者及其家庭的经济负担。到了2024年,梯瓦与江苏恩华药业股份有限公司就安泰坦®达成了在华经销协议,进一步扩大了患者的可及性。可以说,安泰坦®之所以每年都能传来好消息,离不开进博会溢出效应的助力。
今年进博会,安泰坦®缓释片剂Austedo XR®将在中国“首秀”,会进一步提高患者的依从性。此外,梯瓦外用药物密丝高(5%米诺地尔溶液)也将迎来中国“首展”,将丰富中国脱发患者的治疗选择。
依托进博会这一重要桥梁,梯瓦还不断探索数字化生态机会及深化本土合作的机遇。去年,梯瓦积极推动罕见病的治疗信息普及工作,与多方合作提升大众对疾病的认知。通过与阿里、京东、腾讯等平台合作,且联动专家学者完善了HD罕见病地图,上线了TD及HD疾病医典词条,让患者在查询相关信息时得到权威的了解,同时与京东健康开展了提升药物可及性方面的合作。
今年进博会期间,梯瓦还将举行一系列合作签约仪式和医学论坛活动,不断扩大本土“朋友圈”,共同推动行业的高质量发展,打造创新生态。
02
助力健康中国
释放医药“向新力”
“进博会强大的溢出效应远超我们的预期。进博会的强‘磁吸力’让梯瓦能够和全球同行、本土伙伴等深入交流,对于我们深化在华发展具有强大助益。”黄迪仁说,“每年赴约进博会代表着梯瓦重视和深耕中国市场的诚意和决心。”
进入中国以来,梯瓦的中国战略是基于对市场需求的洞察而确定的。过去六年,梯瓦基于市场需求以创新特药为切口进入中国市场,为患者提供创新治疗方案,在中国市场上逐步建立起自己的竞争优势。
创新被梯瓦视为是高质量发展的动力之源。作为百年企业,创新其实根植于梯瓦的品牌基因之中。“梯瓦的仿制药业务备受行业和大众的认可,但大家可能不太了解的是,从1990年代开始,梯瓦已经开始加大全球创新布局,聚焦国际前沿创新领域。例如在帕金森疾病、复发型多发性硬化疾病等方面,我们研发了很多领先且有突破性的治疗方案,为患者带来治疗新选择。”
与此同时,梯瓦一直关注患者所需,助力健康中国发展。近期,进口原研药品的可及性这一问题受到了广泛关注。为了满足中国患者对原研药的需求,今年9月,梯瓦携手海南华蓝医药健康产业有限公司在国内市场上市了舒美特®(阿奇霉素片)。
随着更多创新药物的引进和研发成果的落地,梯瓦希望在中国市场上实现更大的突破和发展。黄迪仁表示,未来,梯瓦将在其长期深耕的中枢神经系统治疗领域向中国加速引进多款创新药。例如,梯瓦计划于2028年引进治疗成人精神分裂症的改良型新药——奥氮平长效针剂,将增加患者治疗的便利度,提升患者的依从性,进一步满足中国患者的健康需求。
03
聚焦创新
深化“在中国,为中国”战略
梯瓦进入中国的六年,正是中国医药行业进入高质量发展新阶段的时间。正如黄迪仁所言,真切感受到了进入中国后的每一步都是机遇。
2018年,梯瓦的肿瘤用药存达®(注射用盐酸苯达莫司汀)国家药监局的批准,这是梯瓦在中国市场迈出的坚实一步。仅仅两年后,治疗舞蹈病及成人迟发性运动障碍的创新药安泰坦®便在华获批,同年更是纳入国家医保目录,极大地提升了药物的可及性,让更多患者受益。随后,梯瓦在进博会的助力下,成功获批神经系统罕见病药物固派松®(醋酸格拉替雷注射液),进一步丰富了产品线。
去年,偏头痛创新药物AJOVY®(瑞玛奈珠单抗)在粤港澳大湾区的落地,得益于“港澳药械通”政策的支持,再次展现了梯瓦在中国市场的敏锐洞察力和强大执行力。对梯瓦而言,除了业务增长和营收提升,最欣慰的是在中国服务患者数量的增加。面对不断变化的市场环境和国家政策的调整,如何在这样一个充满机遇的市场与时俱进,是梯瓦愿意持续探索的问题。
去年,梯瓦提出“Pivot to Growth”全球战略,目的在于“聚焦创新,重启增长之路”。黄迪仁表示,“‘Pivot to Growth’全球战略的推出,是梯瓦主动顺应市场变革,持续巩固市场地位的必然选择。”
值得关注的是,“Pivot to Growth”战略中“创新”不仅体现在创新药业务布局上,还体现在结构、合作方式的创新布局。这一战略由四大增长支柱——发挥增长引擎潜力、夯实创新能力、巩固优势地位、聚焦核心业务,共同构成了梯瓦在中国市场的未来发展蓝图。
作为梯瓦三大国际市场中增长最快的地区之一,梯瓦大中华区将沿袭“Pivot to Growth”战略。黄迪仁强调,“我们注重的是行稳致远的长期效果,希望将更多优质药品及消费品引入中国,并不断提高药品可及性和可负担性,帮助更多患者改善健康状况。”在梯瓦看来,中国医药行业始终展示着巨大的发展潜力。与此同时,日益增长的新型消费趋势也促使梯瓦将好口碑的消费品逐个引入中国市场。
去年,梯瓦引进中国首个健康消费品——Sudocrem®(舒多可蕾)。作为一款源自爱尔兰的经典护臀霜,Sudocrem®畅销全球40多个国家。自去年在京东、天猫海外旗舰店上线以来,该产品的销量与口碑并驾齐驱。在本届进博会上,梯瓦将进一步与浙江英特集团深化合作,扩大该产品的可及性。这是梯瓦在中国多元化战略布局的实施,也是梯瓦深入洞察中国健康消费新趋势和前沿风向的快速落地。
作为一家在华业务迅速增长的中小型跨国药企,梯瓦除了加速全球优质产品的引入,还不断探索最适合自身的发展模式。黄迪仁介绍,梯瓦目前在中国展开了合作销售模式、授权许可模式等多元合作模式,借此来深耕产品与渠道。此外,在团队打造上,旨在构建一个高效敏捷的扁平化组织结构,为业务发展提供了有力保障。
“
对于未来,黄迪仁表示
“梯瓦将会把‘Pivot to Growth’的全球战略与中国处境结合起来,让战略本土化。一方面,梯瓦将会加快创新药物的引进和产品管线布局,丰富患者治疗用药的选择;另一方面,梯瓦将积极与本土企业构建合作关系,加速在企业中国的本土化进展以及影响力。”
审批编号:AUST-CN-00479
生物类似药医药出海
2024-10-27
·信狐药迅
每周药品注册获批数据,分门别类呈现,一目了然。(10.21-10.27)
新药上市申请药品名称企业注册分类受理号替戈拉生片山东罗欣药业集团股份有限公司2.4CXHS2300114替雷利珠单抗注射液勃林格殷格翰生物药业(中国)有限公司2.2CXSS2400013
新药临床申请药品名称企业注册分类受理号TQB3616胶囊正大天晴药业集团股份有限公司1CXHL2400823TQB3616胶囊正大天晴药业集团股份有限公司1CXHL2400822TQB3912片正大天晴药业集团股份有限公司1CXHL2400820注射用SM-V-61上海糖霁生物科技有限公司1CXHL2400766RBD1016注射液苏州瑞博生物技术股份有限公司1CXHL2400235BIOS2210杭州百诚医药科技股份有限公司2.2CXHL2400815BIOS2210杭州百诚医药科技股份有限公司2.2CXHL2400814贝派度酸片甘李药业山东有限公司3CYHL2400161冻干带状疱疹mRNA疫苗武汉瑞佶生物科技有限公司1.2CXSL2400514LVRNA007珠海丽凡达生物技术有限公司1.2CXSL2400512SCTV02注射液神州细胞工程有限公司1.2CXSL2400481四价流感病毒裂解疫苗(ZFA02佐剂)安徽智飞龙科马生物制药有限公司1.3CXSL2400485流感病毒亚单位疫苗(佐剂)江苏中慧元通生物科技股份有限公司1.4CXSL2400493三价流感病毒裂解疫苗(MDCK细胞)成都欧林生物科技股份有限公司2.2CXSL2400430注射用HS-20093上海翰森生物医药科技有限公司1CXSL2400543XKH001注射液浙江鑫康合生物医药科技有限公司1CXSL2400541注射用DB-1305映恩生物制药(苏州)有限公司1CXSL2400537PM8002注射液普米斯生物技术(珠海)有限公司1CXSL2400536注射用BGB-B3227广州百济神州生物制药有限公司1CXSL2400535HD004细胞华道(上海)生物医药有限公司1CXSL2400524SYS6020注射液石药集团中奇制药技术(石家庄)有限公司1CXSL2400511MSCohi-O镜片广东普罗凯融生物医药科技有限公司1CXSL2400496MSCohi-O镜片广东普罗凯融生物医药科技有限公司1CXSL2400495MSCohi-O镜片广东普罗凯融生物医药科技有限公司1CXSL2400494NK510细胞注射液上海贝斯昂科生物科技有限公司1CXSL2400492POV-601-1A1溶瘤病毒注射液苏州般若生物科技有限公司1CXSL2400482阿得贝利单抗注射液上海盛迪医药有限公司2.2CXSL2400538
仿制药申请药品名称企业注册分类受理号注射用盐酸瑞芬太尼江苏恩华药业股份有限公司3CYHS2303613注射用盐酸瑞芬太尼江苏恩华药业股份有限公司3CYHS2303612盐酸丙美卡因滴眼液沈阳兴齐眼药股份有限公司3CYHS2303373氯硝西泮注射液江苏恩华药业股份有限公司3CYHS2301736盐酸美金刚口服溶液温岭市创新生物医药科技股份有限公司3CYHS2301351环丝氨酸胶囊石家庄四药有限公司3CYHS2301331己酮可可碱注射液华夏生生药业(北京)有限公司3CYHS2301197己酮可可碱注射液安徽美来药业股份有限公司3CYHS2301115注射用尼可地尔天大药业(云南)有限公司3CYHS2301036注射用尼可地尔天大药业(云南)有限公司3CYHS2301035己酮可可碱注射液南京卡文迪许生物工程技术有限公司3CYHS2300961磷酸奥司他韦干混悬剂华润三九医药股份有限公司3CYHS2300906琥珀酸美托洛尔缓释胶囊福建省宝诺医药研发有限公司3CYHS2300677生理氯化钠溶液辽宁民康制药有限公司3CYHS2300655生理氯化钠溶液辽宁民康制药有限公司3CYHS2300654注射用吲哚菁绿南京正大天晴制药有限公司3CYHS2300225克林霉素磷酸酯注射液国药集团国瑞药业有限公司3CYHS2300140克林霉素磷酸酯注射液国药集团国瑞药业有限公司3CYHS2300139乙酰半胱氨酸注射液四川汇宇制药股份有限公司3CYHS2201814国;CYHS2201814复方醋酸钠林格注射液仁合益康集团有限公司3CYHS2201740国;CYHS2201740复方醋酸钠林格注射液仁合益康集团有限公司3CYHS2201739国;CYHS2201739卡络磺钠注射液湖北欣泽霏药业有限公司3CYHS2200513国;CYHS2200513注射用头孢唑肟钠广东金城金素制药有限公司4CYHS2400225注射用头孢唑肟钠广东金城金素制药有限公司4CYHS2400224注射用头孢唑肟钠广东金城金素制药有限公司4CYHS2400223普拉洛芬滴眼液合肥利民制药有限公司4CYHS2302244间苯三酚注射液康普药业股份有限公司4CYHS2302141地诺孕素片湖南醇健制药科技有限公司4CYHS2301993艾曲泊帕乙醇胺片宏越科技(湖州)有限公司4CYHS2301969注射用氯诺昔康陕西丽彩药业有限公司4CYHS2301951复方聚乙二醇电解质散(III)成都利尔药业有限公司4CYHS2301884利培酮口腔崩解片兰西哈三联制药有限公司4CYHS2301879注射用头孢唑肟钠成都天之翼尚品医药科技有限公司4CYHS2301859注射用头孢唑肟钠成都天之翼尚品医药科技有限公司4CYHS2301858他达拉非片安徽贝克生物制药有限公司4CYHS2301709他达拉非片安徽贝克生物制药有限公司4CYHS2301708注射用阿糖胞苷四川上善六经医药科技有限公司4CYHS2301707注射用阿糖胞苷四川上善六经医药科技有限公司4CYHS2301706恩格列净片湖南汉森制药股份有限公司4CYHS2301626玻璃酸钠滴眼液吉林敖东药业集团延吉股份有限公司4CYHS2301526盐酸艾司洛尔氯化钠注射液北京百美特生物制药有限公司4CYHS2301401枸橼酸氢钾钠颗粒湖北午时医药研究院有限公司4CYHS2301399来曲唑片北京轩升制药有限公司4CYHS2301264克立硼罗软膏齐鲁制药有限公司4CYHS2301244克立硼罗软膏齐鲁制药有限公司4CYHS2301243精氨酸布洛芬颗粒南京卡文迪许生物工程技术有限公司4CYHS2301222精氨酸布洛芬颗粒南京卡文迪许生物工程技术有限公司4CYHS2301221精氨酸布洛芬颗粒南京卡文迪许生物工程技术有限公司4CYHS2301220盐酸羟考酮缓释片西南药业股份有限公司4CYHS2301181注射用氯诺昔康石家庄四药有限公司4CYHS2301147舒更葡糖钠注射液福建省宝诺医药研发有限公司4CYHS2301132吸入用复方异丙托溴铵溶液成都普什制药有限公司4CYHS2301122间苯三酚注射液四川诺非特生物药业科技有限公司4CYHS2300949注射用厄他培南重庆圣华曦药业股份有限公司4CYHS2300831盐酸乌拉地尔注射液江苏正大丰海制药有限公司4CYHS2300676玻璃酸钠滴眼液浙江尔婴药品有限公司4CYHS2300659玻璃酸钠滴眼液浙江尔婴药品有限公司4CYHS2300658依托咪酯中/长链脂肪乳注射液四川国瑞药业有限责任公司4CYHS2300636盐酸莫西沙星氯化钠注射液江苏睿实生物科技有限公司4CYHS2300575盐酸奥洛他定滴眼液湖北远大天天明制药有限公司4CYHS2300542盐酸奥洛他定滴眼液呋欧医药科技(湖州)有限公司4CYHS2300125卡贝缩宫素注射液成都通德药业有限公司4CYHS2300106普瑞巴林胶囊安徽九洲方圆制药有限公司4CYHS2202083国;CYHS2202083阿加曲班注射液浙江华海药业股份有限公司4CYHS2201603国;CYHS2201603b型流感嗜血杆菌结合疫苗罗益(无锡)生物制药有限公司3.3CXSS2400014托珠单抗注射液金宇博沃润泽生物技术有限公司3.3CXSS2300001
进口申请药品名称企业注册分类受理号尼拉帕利醋酸阿比特龙片Janssen-Cilag International NV2.3JXHS2300035尼拉帕利醋酸阿比特龙片Janssen-Cilag International NV2.3JXHS2300034多种油脂肪乳(C6-24)/氨基酸(16)/葡萄糖(42%)电解质注射液Fresenius Kabi AB5.1JXHS2200008国;JXHS2200008多种油脂肪乳(C6-24)/氨基酸(16)/葡萄糖(42%)电解质注射液Fresenius Kabi AB5.1JXHS2200010国;JXHS2200010多种油脂肪乳(C6-24)/氨基酸(16)/葡萄糖(42%)电解质注射液Fresenius Kabi AB5.1JXHS2200009国;JXHS2200009多种油脂肪乳(C6-24)/氨基酸(16)/葡萄糖(42%)电解质注射液Fresenius Kabi AB5.1JXHS2200011国;JXHS2200011阿卡波糖片STANDARD CHEM.& PHARM.CO.,LTD.5.2JYHS2200002国;JYHS2200002盐酸奎扎替尼片Daiichi Sankyo, Inc.2.4JXHL2400200盐酸奎扎替尼片Daiichi Sankyo, Inc.2.4JXHL2400199CID-103注射液CASI Pharmaceuticals, Inc.1JXSL2400148
中药相关申请
无
注:橙色字体部分结论为不批准或收到通知件;
疫苗临床申请临床1期CSCO会议申请上市
2024-10-27
·药通社
d统计每周仿制药一致性评价通过、上市申请、临床申请及审批情况(10.19-10.25)
注:周统计截止数据定为每周五,周六周日数据将统计入下周,依此类推。
1、仿制药一致性评价过评&驳回情况
本周过评96条(按受理号计算),首仿5家,6个受理号,乙酰半胱氨酸注射液/四川汇宇;生理氯化钠溶液/辽宁民康制药;注射用吲哚菁绿/南京正大天晴;氨甲环酸氯化钠注射液/重庆莱美/赤峰源生。
本周最大话题度的大概就是乙酰半胱氨注射液首仿过评了,过评当天本号推测产品是按照解毒剂过评的,后汇宇官方通告,适应症为急性对乙酰氨基酚中毒解毒,用于预防或减轻其过量引起的肝脏损伤。
拓展阅读:30过1?“乙酰半胱氨酸注射液”首家过评!!
盐酸丙美卡因滴眼液沈阳兴齐眼药的上市申请被驳回,此产品已连续三家被驳回,分别是:合肥立方制药股份有限公司、江西马应龙美康药业有限公司和沈阳兴齐眼药股份有限公司。
兴齐眼药于10月22日发布公告称已撤回盐酸丙美卡因滴眼液的注册申请,兴齐眼药在公告中表示,受政策法规变更等因素影响,结合市场注册申报情况及公司研发策略,经审慎研究,公司决定主动撤回该注册申请。本周沈阳兴齐眼药收到了通知件(为同一受理号)。
注射用吲哚菁绿用于诊断肝硬化、肝纤维化、韧性肝炎、职业和药物中毒性肝病等各种肝脏疾病,了解肝脏的损害程度及其储备功能,也用于脉络膜血管造影,确定脉络膜疾患的位置。南京正大天晴制药是该产品第二家获批的国内药企(首家过评)。
根据摩熵数据显示,2023年在中国公立医院终端诊断用药的销售规模涨至20亿元以上,增长率为15.38%,市场潜力可期。注射用吲哚菁绿该类药物TOP5品种,2023年销售额突破1亿元。
南京正大天晴制药早前暂无诊断用药上市,本次注射用吲哚菁绿成功获批,为公司打开了新市场,正大天晴瞄准诊断用药领域了。
江苏恩华药业过了一个麻精毒放类产品注射用盐酸瑞芬太尼,2023年年销售额达35亿,国内仅有人福医药、国药工业、恩华药业3家企业拥有批文,截止本周此产品的批文都已通过了一致性评价,麻精毒放类药物是暂无集采风险的,任凭集采价格战卷成什么样,都不影响这类拥有麻精毒放药物生产资质的企业。
2、仿制药一致性评价上市申请
3、仿制药一致性评价申请&批准临床
投稿/内容沟通:华籍美人(Ww_150525)
近期精华文章
一致性评价
100 项与 江苏恩华药业股份有限公司 相关的药物交易
登录后查看更多信息
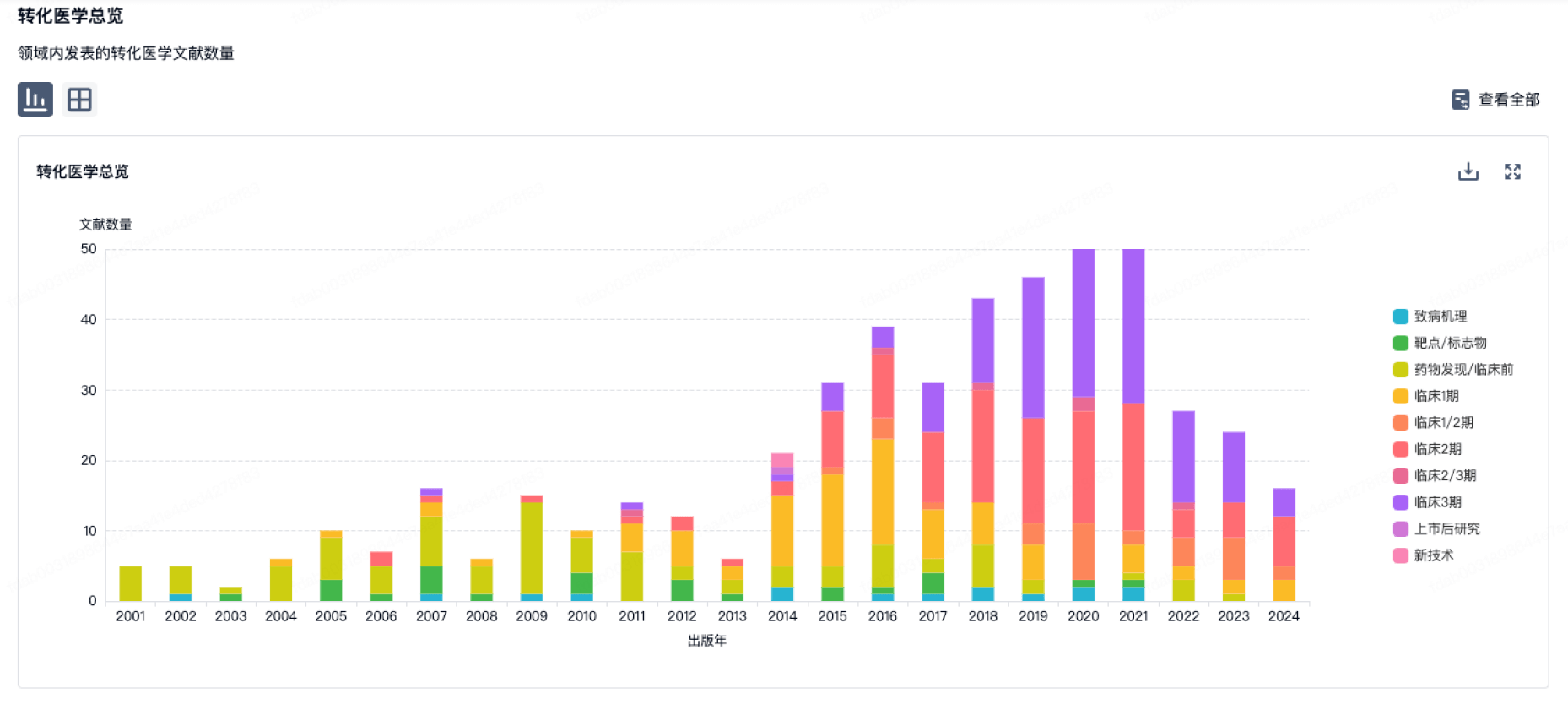
100 项与 江苏恩华药业股份有限公司 相关的转化医学
登录后查看更多信息
组织架构
使用我们的机构树数据加速您的研究。
登录
或
管线布局
2024年11月17日管线快照
管线布局中药物为当前组织机构及其子机构作为药物机构进行统计,早期临床1期并入临床1期,临床1/2期并入临床2期,临床2/3期并入临床3期
药物发现
1
17
临床前
临床申请
2
1
临床申请批准
临床1期
8
3
临床2期
临床3期
3
10
批准上市
其他
10
登录后查看更多信息
当前项目
| 药物(靶点) | 适应症 | 全球最高研发状态 |
|---|---|---|
三唑仑 ( GABAA receptor ) | 入睡和睡眠障碍 更多 | 批准上市 |
依托咪酯 ( GABAA receptor ) | 麻醉 更多 | 批准上市 |
咪达唑仑 ( GABAA receptor ) | 镇静 更多 | 批准上市 |
富马酸奥赛利定 ( μ opioid receptor ) | 急性疼痛 更多 | 批准上市 |
非诺贝特 ( PPARα ) | 高甘油三酯血症 更多 | 批准上市 |
登录后查看更多信息
药物交易
使用我们的药物交易数据加速您的研究。
登录
或

转化医学
使用我们的转化医学数据加速您的研究。
登录
或
营收
使用 Synapse 探索超过 36 万个组织的财务状况。
登录
或
科研基金(NIH)
访问超过 200 万项资助和基金信息,以提升您的研究之旅。
登录
或

投资
深入了解从初创企业到成熟企业的最新公司投资动态。
登录
或

融资
发掘融资趋势以验证和推进您的投资机会。
登录
或

标准版
¥16800
元/账号/年
新药情报库 | 省钱又好用!
立即使用
来和芽仔聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用



