预约演示
更新于:2025-05-07
Fibrous Dysplasia, Polyostotic
多发性骨纤维性发育不良
更新于:2025-05-07
基本信息
别名 ALBRIGHT DISEASE、ALBRIGHT SYNDROME、ALBRIGHT'S SYNDROME + [103] |
简介 FIBROUS DYSPLASIA OF BONE affecting several bones. When melanotic pigmentation (CAFE-AU-LAIT SPOTS) and multiple endocrine hyperfunction are additionally associated it is referred to as Albright syndrome. |
关联
6
项与 多发性骨纤维性发育不良 相关的药物靶点 |
作用机制 IFNγ抑制剂 |
原研机构 |
在研适应症 |
最高研发阶段批准上市 |
首次获批国家/地区 美国 |
首次获批日期2018-11-20 |
靶点 |
作用机制 ERs拮抗剂 |
原研机构 |
最高研发阶段批准上市 |
首次获批国家/地区 美国 |
首次获批日期2002-04-25 |
作用机制 aromatase抑制剂 |
在研机构 |
原研机构 |
在研适应症 |
最高研发阶段批准上市 |
首次获批国家/地区 美国 |
首次获批日期1995-12-27 |
27
项与 多发性骨纤维性发育不良 相关的临床试验ChiCTR2400091528
A prospective, randomized controlled clinical study of the efficacy of denosumab in patients with FD/MAS who are resistant to bisphosphonate therapy
开始日期2024-11-01 |
申办/合作机构 |
NCT06177327
Screening and Epidemiology of Hepato-pancreato-biliary Abnormalities in Fibrous Dysplasia of Bone /McCune Albright Syndrome: the TIM-DYS Study
Fibrous dysplasia of bone /McCune Albright syndrome (FD/MAS) is a rare bone disease caused by somatic mutations in GNAS gene. This GNAS mutation predisposes to cancers, including breast cancer, thyroid cancer, chondrosarcoma and osteosarcoma, as well as biliary tract anomalies, liver-tumors or pancreatic tumors - IPMNs. Intraductal papillary and mucinous neoplasms of the pancreas (IPMN) are cystic intraepithelial ductal lesions developed at the expense of pancreatic ducts. They are pre-cancerous lesions, requiring monitoring and, in case of progression or malignant degeneration, surgical resection. Pancreatic MRI screening of patients with polyostotic FD and MAS is recommended.
The aim of this study is to investigate the epidemiology and characteristics of these hepato-pancreato-biliary abnormalities (prevalence, age of onset, degeneration), based on magnetic resonance imaging (MRI) realized during the follow-up of patients with FD/MAS treated in a French FD expert center.
A better understanding of these IPMNs and other digestive abnormalities will enable clinicians to improve the management and monitoring in this high-risk population.
The aim of this study is to investigate the epidemiology and characteristics of these hepato-pancreato-biliary abnormalities (prevalence, age of onset, degeneration), based on magnetic resonance imaging (MRI) realized during the follow-up of patients with FD/MAS treated in a French FD expert center.
A better understanding of these IPMNs and other digestive abnormalities will enable clinicians to improve the management and monitoring in this high-risk population.
开始日期2024-01-19 |
申办/合作机构 |
ChiCTR2300076039
McCune-Albright syndrome Clinical Registry of China
开始日期2023-10-01 |
申办/合作机构 |
100 项与 多发性骨纤维性发育不良 相关的临床结果
登录后查看更多信息
100 项与 多发性骨纤维性发育不良 相关的转化医学
登录后查看更多信息
0 项与 多发性骨纤维性发育不良 相关的专利(医药)
登录后查看更多信息
3,929
项与 多发性骨纤维性发育不良 相关的文献(医药)2025-07-01·Phytomedicine
Decoding gelsenicine-induced neurotoxicity in mice via metabolomics and network toxicology
Article
作者: Zhai, Jinxiao ; Jiang, Chen ; Ma, Chunling ; Xie, Bing ; Liu, Minghao ; Yan, Hui ; Cong, Bin ; Wen, Di ; Jin, Mingyang
2025-07-01·Fish & Shellfish Immunology
ΔFleQ of Aeromonas hydrophila generated as a live attenuated vaccine in common carp (Cyprinus carpio)
Article
作者: Wang, Kaile ; Fang, Qitong ; Bao, Baolong ; Liu, Binghong ; Nissa, Meher Un ; Che, Jinyuan ; Liu, Zhuochen
2025-06-01·Radiology Case Reports
An atypical multiple autoimmune syndrome: A case report including myocarditis
Article
作者: El Ouafi, Noha ; Ismaili, Nabila ; Achour, Fadoua ; Tahani, Ikram
76
项与 多发性骨纤维性发育不良 相关的新闻(医药)2025-04-09
·梅斯医学
32岁的市场策划经理林蕾(化名)在过去几个月中频繁“高烧不退”,体温动辄飙到40℃以上,吃退烧药也难以缓解。起初她以为只是“反复感冒”,直到伴随关节疼痛、咽痛、皮疹反复发作,连握笔、敲键盘都变得吃力。尤其奇怪的是,每次高烧前后,林蕾的手腕、膝盖、踝关节都出现游走性疼痛,皮肤还会泛出一阵淡粉色的斑疹,下午或晚上尤为明显。公司同事开始议论她“是不是风湿病”,但多次风湿免疫相关指标检查都为阴性。她甚至一度被误认为是精神因素引发的“疑病症”。最终在三甲医院风湿免疫科就诊时,医生发现她白细胞总数高达13×10⁹/L(中性粒细胞占比86%),CRP、ESR、铁蛋白显著升高,肝酶轻度异常,ANA和RF阴性,小剂量抗生素治疗无效。进一步排查排除了感染、肿瘤、自身免疫性疾病等常见原因,结合其长期不明原因高热、典型皮疹、关节痛等表现,最终确诊为成人斯蒂尔病(AOSD)。什么是成人斯蒂尔病成人斯蒂尔病(AOSD)是一种少见的、病因不明的全身性自身炎症性疾病,以发热、皮疹、关节炎或关节痛、咽痛、肝脾及淋巴结肿大、外周血白细胞总数及中性粒细胞比例增高等为主要表现。临床表现成人型Still病(AOSD)临床表现复杂,以发热、关节炎和皮疹为三大典型症状。发热几乎见于所有患者,常为傍晚或夜间骤升至高热,呈弛张热型,常伴寒战,可自行退热且退热后状态良好。关节痛或关节炎多见于2/3以上患者,症状随热程波动,常累及膝、腕等大关节,晚期可致关节破坏。皮疹多为与发热伴发的三文鱼色斑疹或斑丘疹,好发于四肢近端和躯干,亦可见非典型皮损如色素性丘疹、固定性荨麻疹等,提示病情更重、激素反应差。肌痛较为常见,但多无肌酶升高。咽痛常为早期症状,随热程波动,体检可见咽充血、滤泡增生,咽拭子培养阴性。部分患者可有脾大和对称性淋巴结肿大,需鉴别淋巴瘤;肝脏受累者约八成,表现为肝酶升高至严重肝衰,需排除药物因素。心肺受累以浆膜炎和肺实质病变为主,较少见。严重并发症如巨噬细胞活化综合征(MAS)需高度警惕,表现为高热不退、血细胞减少、纤维蛋白原下降等,常因剧烈炎症或继发感染引起。此外,AOSD亦可并发血栓性微血管病、DIC、急性呼吸窘迫综合征等。诊断AOSD缺乏特异性诊断标志,目前主要依赖临床标准进行诊断,其中最常用的是Yamaguchi标准。该标准敏感度达96.3%,特异度为98.2%,前提是排除感染、肿瘤及其他风湿性疾病。Yamaguchi标准包括:主要标准:① ≥39℃发热持续≥1周;② 关节痛持续≥2周;③ 典型皮疹;④ 白细胞≥10×10⁹/L,且中性粒细胞>80%。次要标准:① 咽痛;② 淋巴结或脾肿大;③ 肝功能异常;④ 类风湿因子及抗核抗体阴性。排除标准:感染(如败血症、EB病毒)、恶性肿瘤(如淋巴瘤)、其他风湿病(如系统性血管炎)。诊断成立条件:在排除其他疾病后,符合5项或以上标准,且至少包括2项主要标准,即可诊断AOSD。治疗AOSD治疗以经验性为主,依据病情轻重和是否合并并发症选择方案。轻症可先用非甾体抗炎药,但多数患者疗效有限,仅用于短期对症控制,注意药物不良反应。激素是治疗一线药物,常用泼尼松0.5~1 mg/kg·d 起始治疗,疗效显著,逐步减量,严重病例可考虑甲泼尼龙冲击。对激素无效或减量复发者,应联合DMARDs,首选甲氨蝶呤,其次为环孢素A、来氟米特、羟氯喹等,注意各自副作用。生物制剂为难治性AOSD提供新选择。TNF抑制剂适用于关节炎型AOSD,英夫利西单抗或优于依那西普。IL-6抑制剂托珠单抗可改善全身和关节症状,疗效稳定。IL-1抑制剂如阿那白滞素、卡纳单抗、利纳西普疗效明确,适用于系统型AOSD,卡纳单抗维持时间长,适合激素依赖者。JAK抑制剂如托法替布、巴瑞替尼在小样本中显示出良好疗效,需警惕肝酶和血脂升高等不良反应。静脉注射免疫球蛋白适用于激素依赖或合并MAS等危重患者,可辅助缓解病情,对妊娠期患者亦有益。对难治或合并暴发性肝衰、MAS等严重并发症者,需加大激素剂量,联合两种DMARDs或联合生物制剂、JAK抑制剂,必要时参考HLH-2004方案使用依托泊苷,并可辅以IVIG治疗。参考资料:1.Mitrovic S , Fautrel B . New markers for adult-onset Still′s disease[J]. Joint Bone Spine, 2018,85(3):285-293. DOI: 10.1016/j.jbspin.2017.05.011 .2.朱小霞, 李芹, 王悦, 等. 成人斯蒂尔病诊疗规范[J]. 中华内科杂志, 2022, 61(4): 370-376. DOI: 10.3760/cma.j.cn112138-20211115-00806.3.Hu QY , Zeng T , Sun CY ,et al. Clinical features and current treatments of adult-onset Still′s disease: a multicentre survey of 517 patients in China[J]. Clin Exp Rheumatol, 2019,37Suppl 121(6):52-57.来源 | 梅斯医学编辑 | wanny神经系统罕见病交流群↓点击下方“阅读原文”,下载梅斯医学APP吧!
临床研究
2025-03-26
·美通社
新加坡
2025年3月26日
/美通社/ -- 亚洲领先的生物技术公司
Gene Solutions
宣布在癌症早筛领域取得突破性进展。该公司创新的 SPOT-MAS 检测技术成为亚洲首个完成大规模前瞻性队列验证的多癌筛查血液检测,研究结果已发表于《BMC Medicine》期刊。这一里程碑标志着无创早期检测技术在推动大规模人群应用方面的重大突破。
Gene Solutions 引领癌症早筛新篇章,推出亚洲首个经临床验证多癌血液检测
应对
关
键
医
疗
需求
乳腺癌、结直肠癌等常见癌症若能早期发现,其生存率可超过90%
(
1
)
。然而,在亚洲中低收入国家中超过 70% 的癌症患者被确诊时已处于晚期
(
2
)
。传统的影像学筛查存在诸多局限性,包括侵入性强、可及性低以及单器官系统检测方法导致的高累积假阳性率
(
3
)
。
SPOT-MAS
通过提供可靠、无创的多癌血液检测技术,致力于解决癌症早期发现难题。该技术整合了下一代测序(NGS)技术和人工智能算法,能够检测癌细胞释放到血液中的循环肿瘤DNA(ctDNA)。
在2018至2021年间,SPOT-MAS 完成了实验室技术建立和分析验证。从2022至2024年,SPOT-MAS 检测技术完成了具有里程碑意义的 K-DETEK 临床试验。这是亚洲首个也是规模最大的前瞻性队列癌症筛查研究,共有9,057名无症状人群参与并接受了12个月的随访。经排除特定干扰因素后,最终分析了9,024名参与者的数据,其中 42.27% 因吸烟、饮酒和肝炎病毒感染等因素被归类为高风险人群。
全球第三个、
亚
洲首个大
规
模前瞻性
队
列
临
床
验证
的多癌种早期
检测
(
MCED
)
美国已有两项 MCED 检测产品完成前瞻性验证,但 K-DETEK 临床试验旨在专门为亚洲人群提供了临床数据支撑。尽管全球对MCED测试寄予厚望,但最大挑战之一是从算法模型建立阶段对照试验向真实世界队列研究阶段临床实践过渡时保持一致的高性能。
SPOT-MAS 检测成功应对了这一挑战,各项验证研究阶段中展现一致的性能表现,包括发表于《eLife》上的入组 2,288 人的病例对照研究、发表于《BMC Medicine》的入组 9,024 人的 K-DETEK 临床试验研究,以及发表于 ESMO 肿瘤学年鉴的覆盖东南亚六国(越南、新加坡、马来西亚、泰国、印度尼西亚和菲律宾)10,577 例测试的真实世界经验报告
(
4,5,6
)
。此外,该技术还突显了检测结肠癌前病变的能力,为早期干预和
主
动预
防
结直肠癌创造了机会。
试验
数据亮点
(
5,7
)
:
高灵敏度和特异性:
SPOT-MAS 检测对癌症病变的总体灵敏度达 70.8%,如结合消化道癌前病变评估后
灵敏度
达 78.1%,同时对各类癌症的特异性高达 99.7%。
卓越的
预测
价
值
:
临床试验显示对癌症病例的阳性预测值为 39.53%,如结合癌症和癌前病变后
的
阳性
预测值
达 58.1%。基于算法和机器学习模型的组织溯源,对癌症病变的准确率为 52.9% ,结合癌前病变后
的
准确率可达 84.0%。阴性预测值高达99.92%,确保准确筛查的同时最大限度减少不必要的后续检查。
"SPOT-MAS 是亚洲首个提供癌症筛查真实世界临床证据的 MCED 检测,为区域特定人群提供了必要的验证数据支撑,并有效证明该检测的临床实用性。我们相信,这一重要里程碑将极大促进与区域医疗专家的合作讨论,推动该检测技术纳入常规医疗筛查项目。" Gene Solutions 肿瘤医学总监 MD. PhD.
Nguyen Duy Sinh
表示。
创
新的多
组
学与人工智能整合
与大多数聚焦于循环肿瘤DNA(ctDNA)单一生物标志物的 MCED 测试不同,SPOT-MAS 采用创新的多组学分析方法,整合 ctDNA 的遗传、片段组学和表观遗传学等多重特征分析。这一综合策略极大提升了泛癌覆盖类型及真实世界临床应用中的准确率。
检测技术背后的生物信息学家利用人工智能(AI)分析这些海量的多重特征数据。通过开发多维度机器学习、深度学习和神经网络模型,他们持续使用来自健康个体和癌症患者的标记数据进行深度训练。这种多模态 AI 技术能进一步提高检测灵敏度,准确识别肿瘤组织溯源,并为多组学分析方法优化综合成本效益。
战
略
发
展与合作
创
新
检测在识别缺乏标准筛查方案的致命性癌症方面表现卓越,包括胃癌、肝胆道系统癌症、卵巢癌、胰腺癌、食管癌、子宫内膜癌以及头颈部癌症。同时,它也有效补足现有乳腺癌、结直肠癌和肺癌筛查策略的不足。凭借此次成功临床验证,Gene Solutions 已积极推进 SPOT-MAS 检测技术纳入常规临床实践。公司正与多家顶级医院、监管机构和研究机构开展深度合作,扩大该技术在公立和私立医疗部门的可及性和推广应用。
正如最近接受 GenomeWeb
(8)
采访时,Gene Solutions 研发负责人 Tran Le Son 博士描绘了该项技术的未来发展规划:
持
续进
行和
规
划中的深度研究
:Gene Solutions 正在开展并规划多项延续性研究,包括一项类似 K-DETEK 但聚焦于有癌症疑似症状个体的多中心验证研究。"在这项研究中,我们旨在验证该检测技术在高风险诊断人群中的有效性。我们计划在今年年底前完成研究并提交论文初稿," Tran 博士介绍道。
SPOT-MAS
肺癌和
SPOT-MAS
结
直
肠
癌
检测
:另一项计划在新加坡进行的多中心验证研究将评估 SPOT-MAS 肺癌检测分别在筛查和诊断人群中检测肺癌的有效性。与 SPOT-MAS 泛癌早期检测技术类似,这些单一癌症检测整合分析循环肿瘤DNA(ctDNA)的遗传、片段组学和表观遗传学等多重特征,但特别聚焦于优化识别肺癌或结直肠癌的特异性信号。单一癌症检测方法有望在特定情况下提供显著的筛查性能优势。例如,当强烈怀疑特定癌症时,专为该癌症量身定制的检测可以更具成本效益并提供卓越的性能表现,比普通筛查手段实现更高的灵敏度和特异性。
生物制
药领
域合作
:"展望未来,我们渴望与生物制药公司开展更多合作。" Tran博士表示。Gene Solutions 计划利用其独有人工智能分析平台,基于亚洲为中心的基因组数据优势为个性化癌症治疗和肿瘤疫苗揭示更高效的生物标志物和治疗靶点。
About Gene Solutions
Gene Solutions 是亚洲领先的生物技术公司,专注于利用先进的人工智能和 ctDNA 技术开发创新的癌症检测解决方案。该公司与东南亚超过 4500 家医院和诊所合作,拥有约 250 名生物学专家和技术人员,员工总数超过700 名。Gene Solutions 发表了超过 50 篇同行评审的出版物,并在该地区开展了超过 50 项多中心研究。该公司因其专有研究以及在新加坡和越南获得 CAP 认证的实验室而受到认可,将多组学与人工智能驱动的方法相结合,致力于转变癌症护理模式。
References:
(1) Statistics adapted from the American Cancer Society’s (ACS) publication, Cancer Facts & Figures 2022 and Cancer Facts & Figures 2021; the ACS website; and the International Agency for Cancer Research website.
(2) Sankaranarayanan, R., Ramadas, K., Qiao, Y., 2014. Managing the changing burden of cancer in
Asia
. BMC Med 12, 3.
(3) Imai, M., Nakamura, Y., & Yoshino, T. (2025). Transforming cancer screening: the potential of multi-cancer early detection (MCED) technologies. International Journal of Clinical Oncology.
(4)
Le Son Tran
et al (2023) Multimodal analysis of methylomics and fragmentomics in plasma cell-free DNA for multi-cancer early detection and localization eLife 12:
RP89083
.
(5) Nguyen, L. H. D., et al. (2025). Prospective validation study: a non-invasive circulating tumor DNA-based assay for simultaneous early detection of multiple cancers in asymptomatic adults. BMC Medicine, 23(1).
(6) Annals of Oncology (2024) 35 (suppl_4): S1679-S1697.
(7) Carbonell, Chantelle, et al. Cancer Control 31 (2024).
(8) Genomeweb.com Gene Solutions Looks to Expand MCED Test Market After Strong Validation Data (
19 Feb 2025
).
临床研究基因疗法
2025-02-28
Sobi’s Gamifant was previously approved by the US regulator in 2018. © Swedish Orphan Biovitrum AB.
The US Food and Drug Administration has accepted Sobi’s supplemental biologics licence application for Gamifant (emapalumab-Izsg) for haemophagocytic lymphohistiocytosis (HLH)/macrophage activation syndrome (MAS) associated with Still’s disease.
The application, which seeks approval for use in both adult and paediatric individuals who show an insufficient response or are not tolerant to glucocorticoids, or with recurrent MAS, has received priority review status.
A Prescription Drug User Fee Act date was set by the agency for 27 June 2025.
The sBLA is supported by pooled data outcomes from the EMERALD and NI-0501-06 studies, which enrolled a total of 39 subjects.
53% of subjects achieved a complete response by week 8 and 85% at any time during the studies.
Sobi chief medical officer and research and development head Lydia Abad-Franch stated: “HLH/MAS in Still’s disease is a serious and potentially fatal complication where patients can experience intense hyperinflammation and even multiple organ failure.
“Gamifant (emapalumab-Izsg) selectively neutralises interferon-gamma (IFN-γ), a key driver of hyperinflammation, and if approved, may also help reduce the need for high-dose glucocorticoids in these patients.”
The therapy was previously approved by the US regulator in 2018 to treat primary HLH in adult and paediatric patients, including newborns, with refractory, recurrent or progressive conditions, or those who are not tolerant to conventional HLH therapies.
A critical complication of rheumatic conditions, HLH/MAS is most commonly observed in Still’s disease, including systemic juvenile idiopathic arthritis and adult-onset Still’s disease.
It is marked by severe inflammation, presenting with symptoms such as persistent high fever, elevated levels of ferritin, cytopenias, hepatosplenomegaly and coagulopathies.
The company
received marketing authorisation from the European Commission
in June 2024 for Altuvoct (efanesoctocog alfa), a treatment designed for haemophilia A patients to manage bleeds and provide perioperative prophylaxis.
上市批准临床结果优先审批申请上市
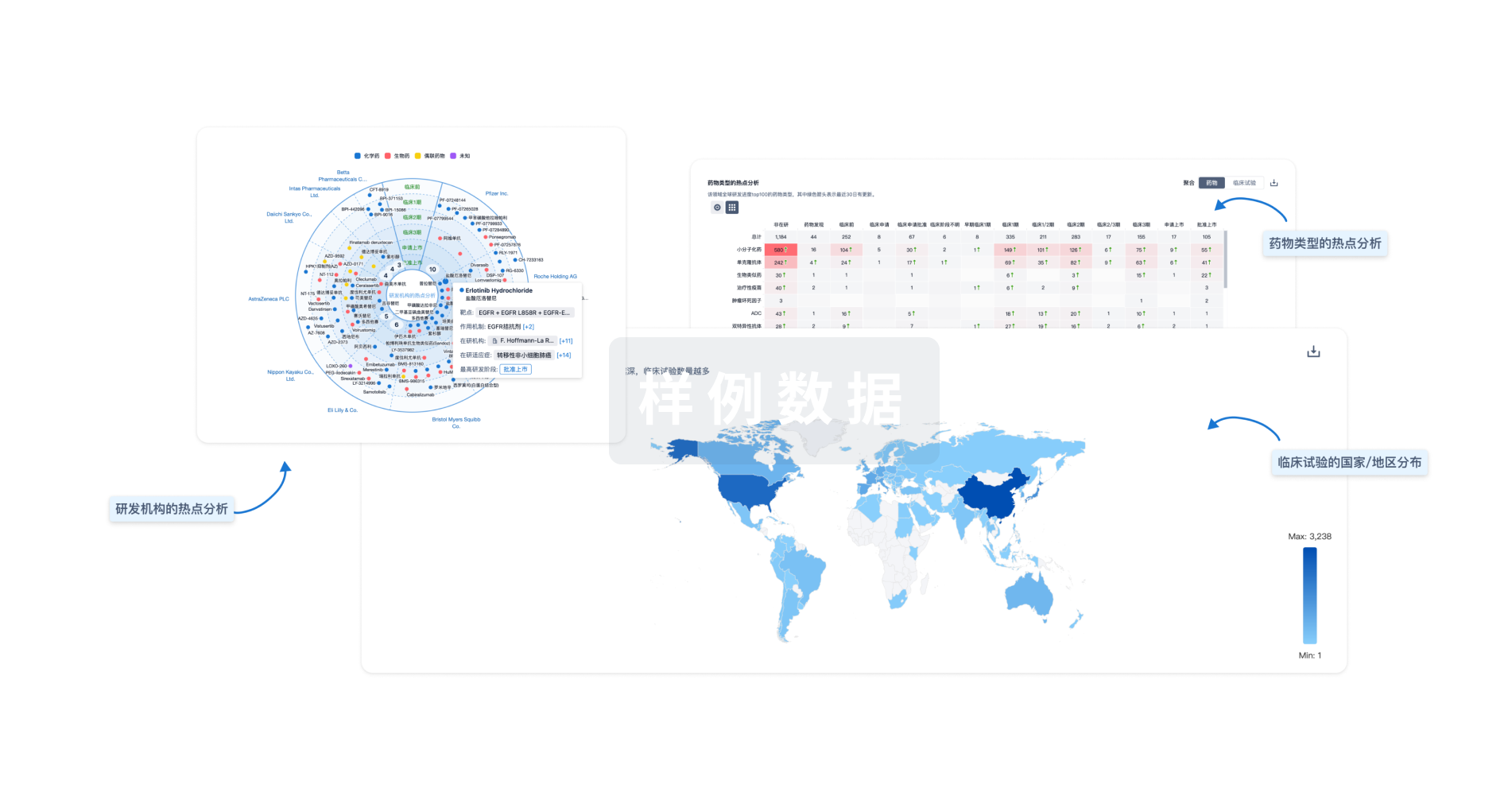
分析
对领域进行一次全面的分析。
登录
或
生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用