预约演示
更新于:2025-05-07
Advanced breast cancer
晚期乳腺癌
更新于:2025-05-07
基本信息
别名 Advanced breast cancer、進行乳癌、シンコウニュウガン |
简介- |
关联
255
项与 晚期乳腺癌 相关的药物作用机制 MEK1抑制剂 [+1] |
在研机构 |
最高研发阶段批准上市 |
首次获批国家/地区 美国 |
首次获批日期2025-02-11 |
作用机制 PARP1抑制剂 [+1] |
在研机构 |
原研机构 |
最高研发阶段批准上市 |
首次获批国家/地区 中国 |
首次获批日期2025-01-14 |
靶点 |
作用机制 HDAC抑制剂 |
原研机构 |
在研适应症 |
最高研发阶段批准上市 |
首次获批国家/地区 中国 |
首次获批日期2024-04-24 |
833
项与 晚期乳腺癌 相关的临床试验NCT06934733
The Efficacy of Oral Cryotherapy in Preventing TROP2-ADC-Induced Oral Mucositis in Patients With Advanced Breast Cancer: A Randomized, Controlled, Multicenter Clinical Study
The incidence of oral mucositis associated with TROP2-ADC therapy in patients with advanced breast cancer is notably high. Identifying effective preventive and therapeutic strategies is essential for enhancing patient quality of life and optimizing treatment outcomes. Oral cryotherapy has been demonstrated to be efficacious in mitigating oral mucositis induced by radiotherapy and chemotherapy. Consequently, this study seeks to investigate the potential preventive efficacy of oral cryotherapy on TROP2-ADC-induced oral mucositis in patients with advanced breast cancer
开始日期2025-05-01 |
申办/合作机构 |
NCT06555588
Engage: A Randomized Controlled Trial Testing the Efficacy of a Telehealth-Delivered Psychosocial Intervention to Decrease Symptom Interference in Patients With Advanced Cancer
The goal of this clinical trial is to test the efficacy a new psychosocial symptom management intervention called ENGAGE for patients with Stage IV breast, prostate, lung, or colorectal cancer. Participants will be randomized to ENGAGE or a Supportive Care intervention. Patient-reported outcomes will be assessed at baseline, 2 months, and 4 months.
开始日期2025-05-01 |
申办/合作机构  Duke University Duke University [+1] |
NCT06486883
A Randomized Phase II Study to Evaluate the Safety and Efficacy of Trastuzumab Deruxtecan Versus CDK4/6 Inhibitor-based Endocrine Therapy as First-line Therapy of HR-positive and HER2-low/Ultralow Advanced Breast Cancer Patients Classified as Non-luminal Subtype According to Gene Expression Profiling.
This trial studies a type of advanced breast cancer defined as hormone receptor HR-positive/HER2-negative and classified as non-luminal by gene expression profiling (PAM50). Patients will be treated with trastuzumab deruxtecan (T-DXd) or with physician's choice of CDK4/6 inhibitor (CDK4/6i) plus endocrine therapy (ET). The main purpose of the study is to analyze the efficacy of T-DXd in patients who have HR-positive and HER2-low/ultralow advanced breast cancer classified as non-luminal subtype.
开始日期2025-05-01 |
100 项与 晚期乳腺癌 相关的临床结果
登录后查看更多信息
100 项与 晚期乳腺癌 相关的转化医学
登录后查看更多信息
0 项与 晚期乳腺癌 相关的专利(医药)
登录后查看更多信息
9,296
项与 晚期乳腺癌 相关的文献(医药)2025-06-01·Critical Reviews in Oncology/Hematology
PARP inhibitors during conception and pregnancy in breast cancer
Review
作者: Andrikopoulou, Angeliki ; Flora, Zagouri ; Dimopoulos, Meletios-Athanasios
2025-06-01·Breast Cancer Research and Treatment
IGF1R activates FOXP3-β-catenin signaling to promote breast cancer development
Article
作者: Luo, Yangkun ; Huang, Na ; Qin, Yuan ; Ren, Jianlan ; Zhang, Zhiming ; Li, Lu
2025-06-01·Breast Cancer Research and Treatment
FGFR4 in endocrine resistance: overexpression and estrogen regulation without direct causative role
Article
作者: Tasdemir, Nilgun ; Levine, Kevin M ; Jankowitz, Rachel ; Dabbs, David ; Hazan, Rachel ; Sikora, Matthew J ; Ding, Kai ; Atkinson, Jenny ; Oesterreich, Steffi ; Chen, Lyuqin ; Shah, Osama ; Lee, Adrian V
1,055
项与 晚期乳腺癌 相关的新闻(医药)2025-05-05
点击上方蓝字 关注我们编者按:在近日举行的2025全国乳腺癌大会上,来自2025年St. Gallen早期乳腺癌共识大会(SGBCC 2025)的专家受邀参会,与中国专家展开了深入的学术交流和讨论,碰撞出许多精彩的火花。《肿瘤瞭望》特邀SGBCC大会主席、奥地利维也纳医科大学综合癌症中心Michael Gnant教授与中山大学孙逸仙纪念医院刘强教授,围绕SGBCC大会投票流程及ctDNA液体活检、遗传性乳腺癌易感基因检测、年轻乳腺癌诊疗等相关热点话题展开对话。The recent 2025 CSCO National Breast Cancer Conference featured an esteemed delegation of experts from the 2025 St. Gallen Early Breast Cancer Consensus Conference (SGBCC 2025), who engaged in profound academic exchanges and discussions with their Chinese counterparts, sparking numerous insightful debates. Ioncology had the privilege of inviting Prof. Michael Gnant, Chair of the SGBCC and Director of the Comprehensive Cancer Center of the Medical University of Vienna, Austria, along with Prof. Qiang Liu from Sun Yat-sen Memorial Hospital of Sun Yat-sen University, to delve into key topics such as the SGBCC voting process, ctDNA liquid biopsy, hereditary breast cancer susceptibility gene testing, and management strategies for young breast cancer patients, offering perspectives rooted in both consensus guidelines and real-world clinical experiences across China and Europe.解密St. Gallen知识转化为患者获益的全过程刘强 教授中山大学孙逸仙纪念医院欢迎Michael Gnant教授再次来到北京,也非常感谢您今天上午在CSCO BC会议上的精彩演讲。我们知道,St. Gallen早期乳腺癌共识会议(SGBCC)多年来在早期乳腺癌治疗的一些争议问题上起到了重要的指引作用。我对每次的会议都非常关注,因为会议每年的主题都会有所不同,投票问题的风格也会发生变化。那么请问,这些年度主题和投票问题是由一个专家小组共同讨论并决定的吗?是否是这样一个机制?Michael Gnant 教授奥地利维也纳医科大学综合癌症中心首先非常感谢邀请我参与采访。我总是很高兴能再回到中国,现在我每年大概会来三到四次。我的太太有时候还会开玩笑说,我们是不是该搬来中国,因为我在这里有很多朋友。同时,CSCO BC会议对于St. Gallen早期乳腺癌共识大会的发展也起到了非常重要的推动作用。您刚刚提出的问题非常有意思,我需要稍微介绍一下背景。SGBCC大会与其他一般的肿瘤学术会议不同,它通常不会发布大量新的临床试验结果,也不像CSCO BC或CACA-CBCS那样即时发布最新的诊疗指南。SGBCC更像是一个基于专家意见的共识形成过程。我常说,这是一个“知识转化为患者获益”的过程。因为无论是临床试验还是指南,归根到底,我们第二天早上在门诊面对患者时,还要做出真正的决策——这就是医学的艺术。那么这个过程是怎么运作的呢?我们在SGBCC会议上努力做到的是:把最新文献和重要学术会议上发布的新研究,通过讨论提炼成日常实践中的可执行方案。而这正是投票的意义所在。我们要回答的问题是:这是否可以成为标准治疗?关于如何制定投票问题,首先有一个由大约10位国际专家组成的核心科学委员会,由来自意大利的Giuseppe Curigliano教授担任委员会主席,他也是ESMO候任主席。还有来自哈佛大学的Harold Burstein教授,他是SGBCC共识讨论的主持人。整个委员会的10位专家来自全球各地,他们会花上两年的时间去搜集可能值得讨论的问题。最近,我们也开始开放给注册参会者提交问题建议,比如:“能不能就这个问题进行投票?”我们通常会从大约150个问题出发,然后通过多轮筛选和集中讨论来精简内容——因为我们没有时间讨论300个问题。随后,还有一个由大约80位专家组成的全体专家团。这一届是历年来人数最多的一次,我们也非常自豪地迎来了5位中国专家首次同时入选投票团。入选专家团投票的问题竞争是非常激烈的。这些专家会在大会前进行预投票。如果某个问题获得100%的一致赞同,我们就不会安排在大会上公开讨论,因为这已经没有争议性了,我们会直接写入后续将发表的共识稿中。而那些结果比较有争议的问题,就会被选出来放到现场进行公开讨论——这正是我们运作的方式。唯一我刚刚还没回答的是:每年大会主题是怎么确定的?其实这确实是个挑战,因为我们每年都希望主题有新意。通常就是我们几个人坐下来,可能开一瓶红酒,聚在一起想出一个最合适的主题——这就是幕后真实的运作过程。滑动查看完整英文Decoding St. Gallen: Translating Knowledge into Patient Benefit – An Exclusive Dialogue with Prof. Michael GnantProf. Qiang Liu:Professor Gnant, welcome back to Beijing. We truly appreciate your excellent presentation this morning at the CSCO BC conference. As we're aware, the SGBCC has played a pivotal role for decades in guiding clinical decision-making for controversial issues in early breast cancer management. I personally follow the conference closely each year, noting how both the annual themes and voting question formats evolve dynamically.Could you walk us through the process of determining these annual themes and voting questions? Is there a dedicated scientific committee that collaboratively develops and finalizes these critical components? What is the exact working mechanism behind this process?Prof. Michael Gnant:First, thank you very much for inviting me to this interview. I'm always delighted to return to China - in fact, I visit about three to four times annually now. My wife sometimes jokes that we should consider moving here, given how many close friends I've made. The CSCO BC conference has also played an instrumental role in advancing the development of the St. Gallen Early Breast Cancer Consensus Conference. Your question touches on something quite fascinating. Let me provide some context. The SGBCC differs fundamentally from conventional oncology conferences - we don't primarily present new clinical trial data, nor do we issue timely treatment guidelines like CSCO BC or CACA-CBCS. Rather, SGBCC represents an expert opinion-driven consensus formation process. I often describe it as "translating knowledge into patient benefit." Because ultimately, whether we're discussing clinical trials or guidelines, we must make real decisions for the patients in our clinics the next morning - that's the art of medicine.So how does this process work? At SGBCC, we strive to distill the latest literature and significant research presented at major conferences into actionable clinical practice recommendations. This is precisely the purpose of our voting process. The core question we address is: Can this become standard of care? Regarding question selection, we have a Scientific Committee comprising about 10 international experts, chaired by Professor Giuseppe Curigliano from Italy (incoming ESMO President), with Professor Harold Burstein from Harvard serving as consensus discussion moderator. This geographically diverse committee spends nearly two years identifying potentially worthwhile discussion topics.Recently, we've opened question submissions to registered attendees. We typically start with about 150 questions, then undergo multiple rounds of rigorous selection - we simply couldn't address 300 questions. The final voting panel consists of approximately 80 experts (our largest ever), including for the first time five distinguished Chinese colleagues. The selection process is highly competitive. These experts conduct pre-meeting voting. Questions achieving 100% consensus are incorporated directly into our published statements, while contentious issues are selected for live debate - that's our operational model. As for annual themes, that's perhaps our greatest challenge. We typically gather over a bottle of wine to brainstorm something fresh and relevant - that's the genuine behind-the-scenes process.从医院走向社区提高乳腺癌早诊早治水平刘强 教授中山大学孙逸仙纪念医院众所周知,SGBCC共识多年来一直在国际上引领着乳腺癌治疗的方向。我印象非常深刻,大概是在12年前,当时我第一次参加SGBCC大会。会议结束回国之后,身边很多同仁都在热烈讨论这一共识:它将如何改变我们的临床工作,它会对我们的日常诊疗带来怎样的影响?可以说,SGBCC共识的确是一项极具影响力的国际性临床共识,也实实在在地惠及了全球范围内的乳腺癌患者。在此,也要衷心祝贺您和您的团队所做出的杰出工作!Michael Gnant 教授奥地利维也纳医科大学综合癌症中心谢谢。如果可以的话,我想稍作补充。当然,并不一定需要完全认同每一项共识的建议,其实我自己也不是每一条都完全赞同。毕竟这本质上是一个投票产生的结果,它更多地反映了全球范围内的意见分布与实际状况。例如,美国的医生在治疗理念上可能和澳大利亚、西班牙,甚至中国的同行都不完全一样。这并不是说哪一方更好或更差,而是不同国家和地区有各自的医学文化与实践背景。但最终,这些观点都需要相互融合、形成共识。就像我最近阅读了中国新发布的CSCO BC和CACA-CBCS指南,我可以看到这些指南在不断地与时俱进、推陈出新,每一次更新都更具现代化,更富有创新性。当然,中国和我的国家那边仍然会存在一些差异。这并不是因为中国的患者有什么不同,或是中国医生不够优秀,而是因为各地患者的分期状况存在差异。我今天早上的演讲中也特别提到,中国其实在很多方面已经取得了令人瞩目的进展。我一直是中国医疗发展的支持者。但中国在早筛和早诊方面仍然面临巨大的挑战。在我的国家,人口总数是900万,可能还不如大连一座城市的人口多。而中国有14亿人口,要实现全国性的筛查项目,难度是可想而知的。不过,随着科技的发展,尤其是人工智能等工具的辅助,我相信中国未来会有更多早期乳腺癌患者被发现。如今,I期乳腺癌的治愈率可达90%。目前中国仍然有很多初诊II至III期的乳腺癌患者。尽管我们拥有了那么多先进的药物和治疗手段,但我们不能忘记,公共卫生的投入仍然是关键。我们必须努力推动筛查、推动早诊早治,让更多患者——大多数是女性,但也不仅限于女性——能够在早期被发现。这样我们的治疗效果才会真正显著提升。滑动查看完整英文From Hospital to Community: Advancing Early Detection and Treatment of Breast CancerProf. Qiang Liu:As we all know, SGBCC has been setting the global standard for breast cancer treatment for many years. In China, I vividly remember attending the SGBCC conference for the first time about 12 years ago. Upon returning, my colleagues and I engaged in passionate discussions about how this consensus would transform our clinical practice and influence our daily patient care. Indeed, the SGBCC represents one of the most impactful international clinical consensuses, delivering tangible benefits to breast cancer patients worldwide. I must sincerely congratulate you and your team on this remarkable achievement.Prof. Michael Gnant:Thank you. If I may, I'd like to add a perspective. It's important to recognize that not every recommendation in the consensus needs to be universally accepted—in fact, I don't personally agree with every single point myself. After all, this is fundamentally a voting-based outcome that reflects the distribution of opinions and practical realities across different regions. For instance, treatment philosophies may differ between physicians in the U.S., Australia, Spain, or even China. This isn't about which approach is superior, but rather about acknowledging diverse medical cultures and practice environments. Ultimately, these perspectives must converge into a unified consensus. I recently reviewed China's updated CSCO BC and CACA-CBCS guidelines and was impressed by their progressive evolution—each iteration more modern and innovative. Of course, differences remain between China and my home country. These variations don't stem from any inherent differences in Chinese patients or any lack of expertise among Chinese physicians, but rather from disparities in disease staging at presentation. In the presentation this morning, I highlighted China's remarkable progress in many areas. Absolutely. I've always been an advocate for China's healthcare advancements. However, significant challenges persist in early screening and diagnosis. My country has a population of 9 million—smaller than Dalian alone—while China must implement nationwide screening for 1.4 billion people. The logistical complexity is staggering. Yet with technological advances, particularly AI-assisted tools, I'm confident China will identify more early-stage breast cancer cases. Today, stage I breast cancer has a 90% cure rate. But China still sees many patients first diagnosed at stages II-III. Despite our advanced therapeutics, we must remember that public health investment remains paramount. We must intensify screening efforts and promote early detection—benefiting primarily women, though not exclusively—to truly transform outcomes.可望但仍有距离方兴未艾的ctDNA临床应用刘强 教授中山大学孙逸仙纪念医院我注意到,在每一届SGBCC会议中,都会不断融入新的内容。例如,今年就专门设立了一个关于循环肿瘤DNA(ctDNA)的专场讨论。我记得在两年前的SGBCC会议上,也曾就ctDNA的相关问题进行了多项投票。目前在我们中心,早期乳腺癌5年生存率已经可以达到90%,而对于整体乳腺癌患者而言,5年乳腺癌特异性生存率也达到了93.2%。我们乳腺肿瘤中心去年就诊的新发乳腺癌患者超过4500例,是中国最大的乳腺癌诊疗中心之一。在这样的高生存率背景下,显然并不需要对每一位患者都采用全覆盖的治疗策略。那么,如何实现更精准、更个体化的治疗,就显得尤为重要。在您看来,ctDNA是否会在未来的精准治疗中发挥关键作用?您认为还有哪些其他技术,可能会在这一方向上发挥主导作用?Michael Gnant 教授奥地利维也纳医科大学综合癌症中心您提出的问题非常重要。我个人必须说,我是ctDNA技术的坚定支持者。但我今年已经 60岁了,从事这个领域的研究工作已有35年。当年我也曾经非常看好ctDNA,但事实证明,这项技术并没有真正落地,效果并不理想。所以,即便我们对ctDNA充满信心,仍然需要有科学证据证明它确实能在乳腺癌治疗中帮助我们实现更精准的决策。那么,我们现在应该如何使用它呢?目前,ctDNA主要用于晚期乳腺癌的液体活检中。我们可以检测ESR1突变,也可能在未来检测PIK3CA突变等。现在我们也在尝试将这项技术应用于早期乳腺癌,比如在辅助治疗阶段做连续监测,希望能把它作为一种超早期的肿瘤标志物。但问题在于:第一,技术成本非常高,如果要每3个月进行一次检测,对于像您中心这样每年有5000名新发患者的机构来说,是几乎不可能完成的。第二,我们仍不确定这种监测是否真正有益。我们当然希望它有用。但假设术后5年,患者的血浆中突然出现了少量ctDNA,我们该怎么办?我们并没有一个明确的答案。所以,在将这类技术真正应用于临床日常实践之前,我们必须通过前瞻性研究证明:根据ctDNA结果做出干预——比如更改治疗方案或添加某种药物——确实能改善患者结局。另一个潜在应用场景是新辅助治疗阶段。我相信,在局部晚期乳腺癌患者中,如果发现患者体内存在ctDNA,我们给予特定的治疗,最终能把血浆中的ctDNA清除,那么这个结果可能成为一个积极的、生物学意义明确的标志物。但同样,我们需要数据支持这一点。最后,从更长远的角度看,我希望未来有一天,我们能够将ctDNA技术用于早期筛查。比如从普通人群中采集血液,比安排所有人去做钼靶或MRI会更容易推广。但这还只是未来的一个愿景,我们还没有达到这个阶段。不过,我对这个方向持非常乐观的态度。也许在10年之内,我们就能推荐将这种技术纳入到乳腺癌的筛查和早诊体系中。刘强 教授中山大学孙逸仙纪念医院谢谢您的分享。确实,如今的技术发展非常迅速。但我们仍需等待更多阳性临床研究的结果,来真正证明我们不仅有能力检测到微小残留病灶,而且可以实实在在地提高高危患者的生存率。尤其是在此前如c-TRAK-TN试验和DETECT-V试验等结果并不理想的背景下,我们更加需要看到证据:这些高风险患者被识别出来之后,我们真的能够改善临床结局,为他们带来生存获益。Michael Gnant 教授奥地利维也纳医科大学综合癌症中心是的,如果我们只是检测,但患者最终还是转移甚至死亡,那还不如节省这笔检测费用。滑动查看完整英文ctDNA in Clinical Practice: Promising Yet Elusive - The Evolving Landscape of Liquid BiopsyProf. Qiang Liu:I've noticed that each SGBCC incorporates new cutting-edge topics. This year featured a dedicated session on circulating tumor DNA (ctDNA), building upon multiple voting discussions about this technology from two years ago. At our center, we've achieved a 90% 5-year survival rate for early-stage breast cancer patients, with the breast cancer-specific survival rate reaching 93.2% overall. As one of China's largest breast cancer centers, we treated over 4,500 new cases last year. With such favorable outcomes, it's clearly unnecessary to adopt a one-size-fits-all treatment approach for every patient. This makes the pursuit of more precise, individualized treatment strategies particularly important. In your view, will ctDNA play a key role in future precision medicine? What other emerging technologies do you think might lead this direction?Prof. Michael Gnant:You've raised a critically important question. Personally, I must say I'm a strong proponent of ctDNA technology. But I'm now 60 years old and have been working in this field for 35 years. I was equally enthusiastic about ctDNA years ago, but the reality is this technology hasn't truly delivered on its clinical promise yet. So while we remain confident about ctDNA's potential, we still need solid scientific evidence proving it can genuinely help us make more precise treatment decisions in breast cancer management. Currently, how should we utilize ctDNA? At present, its primary application is in liquid biopsies for metastatic breast cancer - we can detect ESR1 mutations, and potentially PIK3CA mutations in the future. We're also exploring its use in early-stage breast cancer, such as serial monitoring during adjuvant therapy, hoping to establish it as an ultra-early tumor marker. However, there are significant challenges. First, the technology remains prohibitively expensive. Conducting tests every three months would be nearly impossible to implement at a large center like yours seeing 5,000 new patients annually.Second, we still don't have conclusive evidence that such monitoring provides real clinical benefit. For instance, if we detect trace amounts of ctDNA in a patient's blood five years post-operation, what should we do? We currently lack clear guidelines for such scenarios. Before incorporating this technology into routine clinical practice, we must demonstrate through prospective studies that interventions based on ctDNA results - whether modifying treatment regimens or adding specific medications - actually improve patient outcomes. Another potential application is in the neoadjuvant setting. I believe that for locally advanced breast cancer patients, if we can demonstrate that targeted therapies can eliminate ctDNA from plasma, this could serve as a meaningful biological marker of treatment response. But again, we need robust data to support this approach.Looking further ahead, I'm hopeful that one day ctDNA technology could be used for early detection screening. Blood-based testing would be much easier to implement population-wide than requiring everyone to undergo mammograms or MRI. But this remains a vision for the future - we're not there yet. That said, I'm quite optimistic about this direction. Perhaps within the next decade, we may be able to recommend incorporating this technology into breast cancer screening and early detection programs.Prof. Qiang Liu:Thank you for sharing these insights. Indeed, technological advancements are progressing rapidly. However, we still need to wait for more positive clinical trial results to truly demonstrate that we can not only detect minimal residual disease but actually improve survival outcomes for high-risk patients. Particularly in light of disappointing results from trials like c-TRAK-TN and DETECT-V, we need clear evidence that identifying these high-risk patients translates to meaningful clinical benefit.Prof. Michael Gnant:Exactly. If all we do is detect these molecular signals but patients still experience metastasis or death, then we might as well save the cost of testing altogether. What matters ultimately is whether our interventions based on these sophisticated technologies lead to better patient outcomes. That's the evidence we need to see before widespread clinical adoption.可为和不可为之遗传性乳腺癌易感基因检测刘强 教授中山大学孙逸仙纪念医院谢谢您。第三个问题是,关于您在日常临床实践中开展基因检测的情况。我们知道,遗传检测的类型有很多,包括遗传易感基因检测、BRCA基因检测、以及其他突变检测等。那么关于遗传性检测,您一般是只推荐给年轻患者或有家族史的患者吗?还是说,在临床中有更明确的评估标准来决定是否建议患者进行检测?Michael Gnant 教授奥地利维也纳医科大学综合癌症中心关于乳腺癌遗传性风险或高风险人群的基因检测,我认为当前的情况已经发生了一些变化,尤其是在2025年SGBCC大会之后。过去,我通常只会为非常年轻的患者,或者有乳腺癌或卵巢癌强家族史的患者安排相关检测。但在今年的SGBCC大会上,针对这一问题有一些非常具体的投票。首先的问题:是否应该对所有乳腺癌患者进行遗传检测? 答案非常明确:不需要。接下来的问题是:是否应该为70岁以下的所有患者进行检测?答案依然很清楚:不建议,因为工作量太大,检出率太低,获益有限。不过,当问到:是否应该为50岁以下的乳腺癌患者进行检测?有不少专家倾向于建议进行检测。而对于三阴性乳腺癌患者,今年的投票中也显示出一个新的趋势:无论年龄大小,都建议进行遗传检测,这是一个比较新的共识变化。所以如果遇到三阴性乳腺癌患者,我们应该考虑为她本人以及其家庭成员提供遗传检测,这可能是有益的。我认为我们正朝着更多开展遗传检测的方向发展。但我们也必须意识到,基因检测可能会引发患者的焦虑和恐惧,同时也存在经济负担等现实问题。因此,并不是所有人都适合做检测。有时我们可能会发现一些临床意义尚不明确的突变。但对于BRCA1和BRCA2基因突变——以及未来可能更多涉及的PALB2突变,如果患者年龄在50岁以下,或者是三阴性乳腺癌患者,我认为更常规地开展检测是一个不错的选择,因为我知道在中国,有相当多的乳腺癌患者是绝经前人群,因此这一部分人群可能尤为值得关注和覆盖。滑动查看完整英文Hereditary Breast Cancer Genetic Testing: Navigating the Boundaries of Clinical UtilityProf. Qiang Liu:Thank you. My third question focuses on your clinical approach to genetic testing. We're aware there are multiple testing modalities available - from hereditary predisposition panels to targeted BRCA analysis and broader mutational profiling. In your practice, do you reserve genetic testing primarily for younger patients or those with strong family histories? Or do you employ more comprehensive clinical criteria when determining testing eligibility?Prof. Michael Gnant:The paradigm for genetic testing in hereditary breast cancer risk assessment has undergone significant transformation, particularly following the 2025 St. Gallen consensus. Historically, my testing criteria were restricted to two clear cohorts: first, exceptionally young patients, and second, those with compelling family histories of breast or ovarian malignancies. However, the recent SGBCC deliberations have reshaped our clinical approach through several pivotal consensus votes. Let me outline the key determinations: First, the fundamental question: Should we implement universal genetic testing for all breast cancer patients?The assembled experts reached a definitive conclusion. Routine population-wide testing is not clinically justified.Second: Should we extend testing to all patients under 70 years?The consensus remained clear. This approach is not recommended due to three critical limitations - excessive clinical burden, unacceptably low detection rates, and marginal improvement in clinical outcomes.However, the discussion revealed important nuances. For patients under 50, we observed growing expert consensus favoring more liberal testing policies.Most significantly, we identified a paradigm shift regarding triple-negative breast cancer (TNBC). The current consensus recommends genetic testing for all TNBC patients regardless of age - representing a substantial evolution in our clinical guidelines. This means that for any TNBC diagnosis, we should now consider comprehensive genetic evaluation of both the index patient and relevant family members. While this represents progress toward broader testing adoption, we must remain cognizant of several practical considerations. Genetic testing can induce significant patient anxiety and carries non-trivial economic implications. Moreover, we frequently encounter variants of uncertain significance (VUS) that create clinical dilemmas without clear resolution pathways.For clearly actionable mutations - particularly BRCA1/2 and increasingly PALB2 - I advocate for systematic testing in two key populations: All breast cancer patients under 50 years OR all triple-negative cases regardless of age. This approach holds particular relevance for China's unique epidemiological landscape, where premenopausal breast cancer represents a substantial proportion of cases. The potential clinical impact in this population makes genetic evaluation especially compelling.无过度无不及年轻乳腺癌的规范化诊疗刘强 教授中山大学孙逸仙纪念医院我们其实也在向这一趋势发展,如今开展遗传检测的频率明显比往年提高了很多。大家逐渐意识到,至少有5%到10%的乳腺癌是遗传性的。所以,了解发病原因,以及是否存在特定基因突变,不仅有助于制定个体化的治疗方案,也对未来癌症的预防具有重要意义。Michael Gnant 教授奥地利维也纳医科大学综合癌症中心是的,不过我认为我们也需要意识到,即使检测出更多的基因突变,我们也不应该给患者施加压力,去做双侧乳房切除等激进治疗。这应该是一个共同决策的过程。对于一个健康个体来说,她应该在接受充分的专业咨询后,自己决定是否选择更密集的监测——比如比一般人更频繁地做核磁共振,或者选择进行降低风险的手术干预。而这类决策往往依赖于患者的年龄、家庭状况及其他多个因素。我个人并不赞同所有携带突变的人都应该接受乳房切除术的趋势。同时我们也要意识到,从预防性手术中获益最大的,其实是健康人群。而对于已经患有乳腺癌的患者来说,再做预防性的对侧乳房切除手术,带来的实际益处可能非常有限。因为对这些患者而言,真正影响预后的往往是原发病灶所导致的转移,而不是对侧乳房是否患癌。这是我们在医学界内部需要清晰传达的信息,同时也需要与患者明确沟通。这也是为什么我认为,这类问题应该在有经验的乳腺专科中心由专业人员来处理,而不是由所有机构都随意提供基因检测服务。否则患者可能会因为信息不足而做出对自己来说并不合适的选择。刘强 教授中山大学孙逸仙纪念医院我完全同意您的观点。在中国,您也知道,年轻的乳腺癌患者非常多。比如在我们乳腺肿瘤中心,将近20%的患者年龄在40岁以下,其中有9.4%的患者年龄在35岁以下,这些都非常年轻。就像上周,我门诊中就接诊了一位21岁和一位23岁的乳腺癌患者,真的非常年轻。在您的临床实践中,也会遇到这么年轻的乳腺癌患者吗?Michael Gnant 教授奥地利维也纳医科大学综合癌症中心我们偶尔会遇到这么年轻的患者,但没有中国这么常见。我接诊过的最年轻乳腺癌患者是18岁,但这是在多年来接诊的8000多位患者中的个例。在中欧地区,年轻乳腺癌患者的比例明显低于亚洲,也低于中国。不过,我认为,只要发现得够早,即使在35岁确诊乳腺癌,也完全可以做到规范有效的治疗。我记得是昨天还是前天,我还在门诊协助同事进行会诊,当时见到了不少年轻患者。但在我平时的临床工作中,大约四分之三的患者都是绝经后的女性,所以我们接触到的年轻患者相对较少。我个人认为,从生物学特征上来看,年轻患者和年长患者之间并没有太大差异。真正的区别可能体现在:筛查的可及性、早诊的能力、以及大众的认知水平。比如在我们国家,三四十年前,在一些农村地区的情况也非常不理想,那时候筛查体系几乎是空白。所以我们花了很多年才逐步建立起现在的体系。到去年为止,我们国家95%以上的乳腺癌患者都在认证的乳腺癌中心接受治疗,这是第一次达到这样的比例。但这并不是从一开始就有的,这套体系是我们过去二十年逐步建设起来的成果。我相信中国也一定会发展出同样成熟、完善的体系。我对此非常乐观。刘强 教授中山大学孙逸仙纪念医院如今,我们对于年轻女性乳腺癌的关注程度已经大大提升。在中国,我们制定了《中国年轻乳腺癌诊疗专家共识》,我本人是该共识的发起人。去年,我们也召开了第二次专家会议,就针对这类患者的一些关键问题进行了专题投票讨论。我完全认同您的观点,即预防性乳房切除术应基于医生与患者之间的共同决策。尤其是在中国,BRCA突变并没有明确的乳腺癌高发热点突变区域。即使患者存在明确的致病性BRCA突变,其家族史表现也可以差异非常大。比如上个月我接诊的一位患者,她有6个姐妹,其中有4位曾患乳腺癌或卵巢癌,这是一个非常强的家族遗传倾向。但也有很多患者,虽然携带了明确的致病突变,却没有任何家族史。所以即便是同样的BRCA突变,其外显率在不同个体之间差异也可能非常大。因此,我们在临床决策中必须综合考虑:患者的家族史、个人偏好、以及其他临床和社会因素,来制定最合适的管理策略。Michael Gnant 教授奥地利维也纳医科大学综合癌症中心我完全赞同您的观点。刘强 教授中山大学孙逸仙纪念医院非常感谢您。今天我们和Gnant医生就圣加仑共识的最新进展、遗传检测的临床应用,以及年轻乳腺癌患者的管理等内容,进行了非常深入的交流。十分感谢您的分享。Michael Gnant 教授奥地利维也纳医科大学综合癌症中心很荣幸能参与今天的讨论,感谢邀请。滑动查看完整英文Precision in Practice: Navigating the Complexities of Young Breast Cancer ManagementProf. Qiang Liu:We're certainly moving toward broader adoption of genetic testing, with significantly increased testing rates compared to previous years. There's growing recognition that 5-10% of breast cancers are hereditary. Identifying these genetic drivers informs both personalized treatment and future cancer prevention strategies.Prof. Michael Gnant:Absolutely. However, we must emphasize that detecting mutations shouldn't automatically lead to radical interventions like bilateral mastectomies. These decisions require shared decision-making. A healthy mutation carrier, after thorough counseling, should determine her own surveillance plan—whether more frequent MRIs or risk-reducing surgery—based on age, family circumstances, and other factors. I'm concerned about the trend toward universal prophylactic surgery. The greatest benefit actually accrues to unaffected individuals. For breast cancer patients, contralateral mastectomy offers limited survival advantage since prognosis is typically driven by metastatic potential of the primary tumor, not contralateral disease. This nuanced message must be clearly communicated both within the medical community and to patients. That's why I advocate restricting genetic services to specialized breast centers where experts can provide balanced counseling—not through indiscriminate testing programs that may lead to uninformed choices.Prof. Qiang Liu:I completely agree. In China, we see exceptionally high rates of young-onset breast cancer. At our center, 20% of patients are under 40, including 9.4% under 35—extraordinarily young. Just last week, I diagnosed 21- and 23-year-old patients. Do you encounter such young cases in your practice?Prof. Michael Gnant:Occasionally, but not as frequently. My youngest patient was 18, but she represented one case among 8,000+ treated over decades. Central Europe's young-onset incidence is markedly lower than Asia's. However, with early detection, even a 35-year-old diagnosis can be managed effectively. Actually, just yesterday - or was it the day before? - I was consulting on a case in clinic and saw several young patients. But to put this in context, postmenopausal women make up about three-quarters of my regular caseload, so younger patients are relatively uncommon in my practice.Biologically, tumors in young versus older patients show minimal differences. The real disparities lie in screening access, diagnostic capabilities, and public awareness. Our own rural areas had virtually no screening infrastructure 30-40 years ago. Building our current system—where 95% of patients now receive care at certified centers—took two decades of sustained effort. I'm confident China will develop similarly robust systems.Prof. Qiang Liu:China has significantly elevated focus on young breast cancer. We've established the Chinese Expert Consensus on Young Breast Cancer, which I chaired. Last year's consensus meeting featured dedicated voting on key management questions.Your perspective on shared decision-making for prophylactic surgery resonates strongly. Notably, China lacks BRCA mutation hotspots—even pathogenic variants demonstrate highly variable penetrance. Let me give you a concrete example - just last month, I counseled a patient with six sisters, four of whom had been diagnosed with either breast or ovarian cancer. That's a textbook example of strong familial predisposition. But here's the clinical reality we often face: many confirmed mutation carriers present with no family history whatsoever. This highlights the remarkable variability in BRCA penetrance, even with identical mutations. Thus, clinical decisions must integrate family history, patient preferences, and psychosocial factors.Prof. Michael Gnant:Fully concur.Prof. Qiang Liu:Thank you, Professor Gnant, for this profound discussion on St. Gallen updates, genetic testing applications, and young breast cancer management.Prof. Michael Gnant:The honor was mine. Thank you for the invitation.刘强 教授教授、主任医师、研究员、博士生导师中山大学孙逸仙纪念医院外科主任逸仙乳腺肿瘤医院执行副院长、乳腺肿瘤中心主任、乳腺外科主任ESO-ESMO年轻乳腺癌国际共识专家组成员中国临床肿瘤学会乳腺癌专业委员会常务委员兼副秘书长中国抗癌协会乳腺癌专业委员会常务委员中国抗癌协会肿瘤分子医学专业委员会常务委员广东省医学会乳腺病分会主任委员广东省抗癌协会乳腺癌专业委员会主任委员中国普通外科杂志副主编和中华内分泌外科杂志副总编辑新加坡国立大学外科博士,回国前任哈佛大学Dana Farber癌症中心讲师主持多项国家级重大项目包括多项国自然重点项目和国际合作重点课题,率先开展液体活检和免疫联合治疗在乳腺癌的应用,并发起和制定中国首部年轻乳腺癌诊疗专家共识,2020年获人民日报社主办的“国之名医·优秀风范”荣誉称号。擅长乳腺癌的诊断、手术和综合治疗,尤其高难度保乳手术、年轻乳腺癌和三阴乳腺癌的个体化精准治疗。Michael Gnant 教授奥地利维也纳医科大学综合癌症中心外科学教授奥地利乳腺癌和结肠直肠癌研究小组主席(来源:《肿瘤瞭望》编辑部)声 明凡署名原创的文章版权属《肿瘤瞭望》所有,欢迎分享、转载。本文仅供医疗卫生专业人士了解最新医药资讯参考使用,不代表本平台观点。该等信息不能以任何方式取代专业的医疗指导,也不应被视为诊疗建议,如果该信息被用于资讯以外的目的,本站及作者不承担相关责任。
CSCO会议
2025-05-01
点击蓝字,关注我们编者按:近年来,PAM信号通路的相关研究进展不断,随着PI3Kα抑制剂(伊那利塞)和AKT抑制剂(卡匹色替)等药物在中国相继获批上市,PAM通路抑制剂相关诊疗药物备受瞩目。在St.Gallen国际乳腺癌大会中国行南京站上,江苏省人民医院李薇教授带来了“HR阳性晚期乳腺癌PAM诊疗之路”的讲课,会后,肿瘤瞭望特邀请李薇教授就HR阳性晚期乳腺癌患者的诊疗现状与挑战,PAM通路抑制剂在精准治疗中的重要性及其临床应用前景进行了介绍。01肿瘤瞭望:您在本次大会上带来了“HR阳性晚期乳腺癌PAM诊疗之路”的讲课,首先,请您为我们介绍一下HR阳性晚期乳腺癌患者的诊疗现状与面临的挑战。李薇教授:目前,在乳腺癌患者群体中,激素受体阳性(HR+)晚期乳腺癌患者占比较大。在诊疗过程中,既往针对这部分患者我们拥有多种单药内分泌治疗方案可供选择。随着CDK4/6抑制剂、PAM通路抑制剂进入临床应用,确实为这部分患者增添了更多可选的治疗方案,同时也使患者获得了更长的生存获益。在CDK4/6抑制剂广泛应用的当下,我们在努力提升HR+乳腺癌患者生存率的同时,也发现了诸多亟待解决的问题。其中,在CDK4/6抑制剂治疗进展后,如何选择后续治疗药物是一个关键问题。目前,针对这部分患者,CSCO BC诊疗指南并未给出明确的I级推荐方案,可供选择的治疗方案包括:CDK4/6抑制剂跨线联合内分泌治疗;其他靶向治疗药物联合内分泌治疗;ADC药物治疗以及参与临床研究等。在此背景下,对这些患者进行精准的治疗区分至关重要。例如,对于存在PAM信号通路突变的患者,PAM通路抑制剂是更优的治疗选择;对于BRCA突变的患者,PARP抑制剂则是很好的选择。总之,对于HR+晚期乳腺癌患者而言,在CDK4/6抑制剂治疗进展后,精准区分患者人群并进行精准检测是非常关键的。02肿瘤瞭望:近年来PAM通路抑制剂在乳腺癌精准治疗中备受关注,请结合最新诊疗进展和2025版CSCO BC指南更新情况,谈谈其对HR阳性晚期乳腺癌的重要价值。李薇教授:2025年开年之后,已有两个PAM通路抑制剂相继获批上市,分别为PI3Kα抑制剂(伊那利塞)和AKT抑制剂(卡匹色替),因此,PAM通路抑制剂的关注度持续攀升。可以预见,随着更多此类药物的获批,它们将逐步应用于临床。因此,PAM通路抑制剂的临床应用已成为当前关注的热点。在应用PAM通路抑制剂之前,我们需要对患者进行精准检测,以确定其是否存在PIK3CA/AKT1/PTEN基因突变或缺失,从而确保这部分患者能够接受更为精准的治疗。在检测过程中,我们可以选择组织样本或血液样本进行检测,也可以选择对转移灶或原发灶进行检测。对于PAM信号通路中存在基因突变或缺失的患者而言,无论选择对转移灶还是原发灶进行检测,或者采用组织学还是血液学检测方法,其结果差异并不显著。因此,在整个检测过程中,操作相对便捷。我们应将精准检测的理念在患者中进行推广,使其深入人心,让更多患者接受精准检测,进而获得更为精准的治疗。03肿瘤瞭望:精准检测是精准治疗的重要前提,针对HR阳性晚期乳腺癌PAM诊疗之路,需要怎样的检测体系支撑?这些技术如何帮助患者实现真正的个体化治疗决策?李薇教授:刚才谈到,精准检测是HR+晚期乳腺癌患者开展治疗的前提,在患者开始治疗前,需将有相关突变的患者区分出来。以今年获批的药物为例,伊那利塞的获批适应症为:联合哌柏西利和氟维司群,适用于内分泌治疗耐药(包括在辅助内分泌治疗期间或之后出现复发)、PIK3CA突变、激素受体(HR)阳性、人表皮生长因子受体2(HER2)阴性的局部晚期或转移性乳腺癌成人患者。基于此,我认为针对这部分患者应尽早开展相关检测。对于患者手术后的标本,也可进行相关基因检测。如此一来,一旦患者出现病情进展,便能有更为精准的诊疗方案。所以,若患者条件允许,应尽早进行检测。获得患者手术标本或血液组织标本等检测结果后,对后续治疗将起到很好的指导作用。李薇 教授江苏省人民医院 肿瘤科博士 主任医师 副教授 硕士生导师中国临床肿瘤学会(CSCO)乳腺癌专家委员会委员中国临床肿瘤学会(CSCO)青年专家委员会常委中国抗癌协会乳腺癌专业委员会委员中国抗癌协会肿瘤靶向治疗专业委员会委员长江学术带乳腺联盟(YBCSG)副主任委员江苏省研究型医院肿瘤分子靶向治疗专业委员会常委江苏省抗癌协会癌症康复与姑息治疗专业委员会委员江苏省“科教强卫工程”青年人才美国南卡莱罗那大学医学院访问学者(来源:《肿瘤瞭望》编辑部)声 明凡署名原创的文章版权属《肿瘤瞭望》所有,欢迎分享、转载。本文仅供医疗卫生专业人士了解最新医药资讯参考使用,不代表本平台观点。该等信息不能以任何方式取代专业的医疗指导,也不应被视为诊疗建议,如果该信息被用于资讯以外的目的,本站及作者不承担相关责任。
CSCO会议上市批准抗体药物偶联物临床研究临床1期
2025-04-30
·癌度
癌度临床试验病友交流群(点击)一、新药快讯1、抗体偶联药联合PD-1单抗让80%患者病灶缩小30%2025年美国癌症研究协会AACR年会在芝加哥召开,来自中山大学肿瘤防治中心徐瑞华教授团队报道了一项研究,靶向CLDN18.2的抗体偶联药JS107治疗晚期实体瘤的一期临床试验结果,JS107联合PD-1抗体特瑞普利单抗对于既往未接受治疗的晚期胃癌或胃食管结合部腺癌,治疗应答率达到了81%,治疗效果与CLDN18.2表达水平正相关。2、泽妥珠单抗治疗NRG1融合阳性非小细胞肺癌,刊登新英格兰医学泽妥珠单抗是一种双靶点抗体药,可以同时靶向HER2和HER3,2025年2月新英格兰医学刊登了一项二期临床试验,对于存在NRG1融合突变的非小细胞肺癌、胰腺癌等12种实体瘤,30%的患者可评估肿瘤病灶缩小超过30%,有1名患者完全缓解,中位疾病进展时间为11.1个月,目前该药已经获得2025版本CSCO指南推荐。3、美国前沿药Zoldonrasib治疗KRAS基因G12D肺癌展现惊人疗效在2025年美国癌症研究协会AACR年会上,一项一期临床试验结果表明,前沿药Zoldonrasib(代号RMC-9805)在携带KRAS基因G12D突变的非小细胞肺癌患者中展现出积极抗肿瘤活性,且耐受性良好。每日1200毫克剂量组观察到61%的患者肿瘤病灶显著缩小或完全消失,疾病控制率达到了89%,平均治疗时间为2.6个月。二、抗癌前沿1、癌症治疗疫苗Bria-IMT联合免疫治疗为晚期乳腺癌带来新希望2025年美国癌症研究协会AACR年会上,一项早期人体临床试验显示,癌症疫苗Bria-IMT联合免疫检查点抑制剂,在经过多次治疗的转移性乳腺癌患者中展现出良好的安全性、耐受性及临床获益证据。42名患者有10%出现了病灶缩小超过30%或完全消失,临床获益率达到了55%;HER2阳性乳腺癌治疗应答率达到了50%,临床获益率达到了100%;激素受体阳性且HER2阳性乳腺癌治疗应答率为10%,临床获益率达到了55%。三阴乳腺癌无治疗应答,临床获益率为45%。
AACR会议临床结果疫苗临床1期
分析
对领域进行一次全面的分析。
登录
或
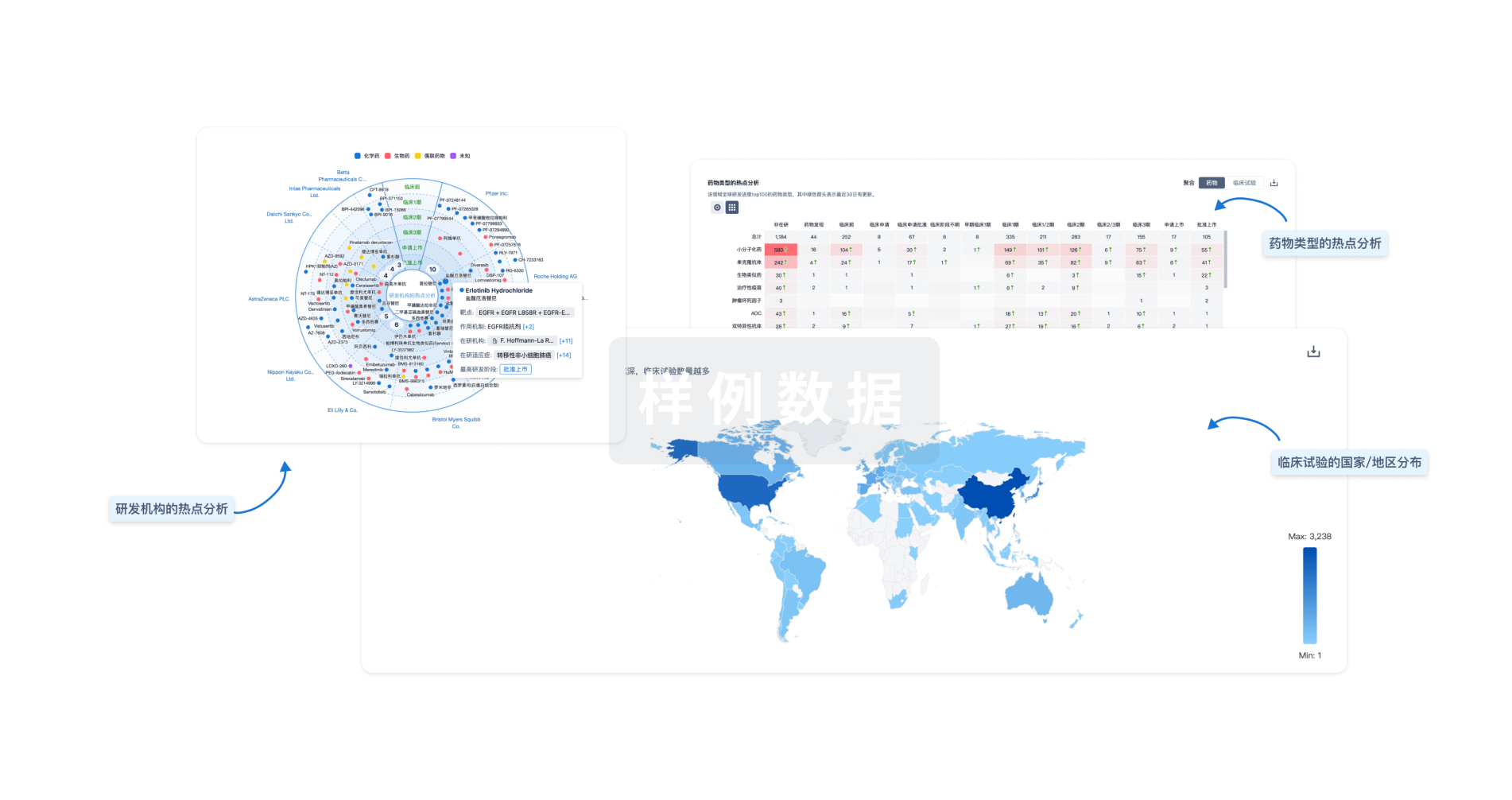
Eureka LS:
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用






