预约演示
更新于:2025-05-07
Carotid Artery Diseases
颈动脉疾病
更新于:2025-05-07
基本信息
别名 Arterial Disease, Carotid、Arterial Diseases, Carotid、Arterial Diseases, Common Carotid + [61] |
简介 Pathological conditions involving the CAROTID ARTERIES, including the common, internal, and external carotid arteries. ATHEROSCLEROSIS and TRAUMA are relatively frequent causes of carotid artery pathology. |
关联
29
项与 颈动脉疾病 相关的药物靶点 |
作用机制 PCSK9抑制剂 |
原研机构 |
在研适应症 |
最高研发阶段批准上市 |
首次获批国家/地区 美国 |
首次获批日期2015-07-24 |
作用机制 脂质调节剂 [+1] |
在研机构 |
原研机构 |
最高研发阶段批准上市 |
首次获批国家/地区 美国 |
首次获批日期2004-11-10 |
靶点- |
作用机制- |
原研机构 |
最高研发阶段批准上市 |
首次获批国家/地区 中国 |
首次获批日期2003-02-13 |
1,016
项与 颈动脉疾病 相关的临床试验NCT06714097
Application of Digital Twins' Technology in Patients Who Had a Stroke, with Moyamoya Disease and with Cerebral Amyloid Angiopathy (CAA) During the Secondary Prevention Phase: a Proof of Concept Using a Randomized Control Trial (Clinical Study 6, STRATIF-AI Project)
The goal of this clinical trial is to obtain initial feedback on the implementation of the STRATIF-AI platform and digital twin in the secondary prevention phase in patients with stroke, Moyamoya disease and Cerebral amyloid angiopathy, including adults of both sexes.
The main questions it aims to answer is: how is the patients' experience regarding the use of the STRATIF-AI platform? Researchers will compare Arm 1, who use the STRATIF-AI app in addition to standard secondary prevention, against Arm 2, who receive standard secondary prevention only, to collect feedback regarding the platform usability.
Participants in the Arm 1 will:
* Complete cognitive and psychological assessments at the time of the first visit and after six months
* Follow the indications received from the clinician for standard secondary prevention
* Use the STRATIF-AI app daily for health management
* Optionally, purchase wearable devices that connect to the app.
* Participate in interviews at the six-month mark to share their experiences with the app.
Patients in the Arm 2 will:
* Complete cognitive and psychological assessments at the time of the first visit and after six months
* Follow the indications received from the clinician for standard secondary prevention
The main questions it aims to answer is: how is the patients' experience regarding the use of the STRATIF-AI platform? Researchers will compare Arm 1, who use the STRATIF-AI app in addition to standard secondary prevention, against Arm 2, who receive standard secondary prevention only, to collect feedback regarding the platform usability.
Participants in the Arm 1 will:
* Complete cognitive and psychological assessments at the time of the first visit and after six months
* Follow the indications received from the clinician for standard secondary prevention
* Use the STRATIF-AI app daily for health management
* Optionally, purchase wearable devices that connect to the app.
* Participate in interviews at the six-month mark to share their experiences with the app.
Patients in the Arm 2 will:
* Complete cognitive and psychological assessments at the time of the first visit and after six months
* Follow the indications received from the clinician for standard secondary prevention
开始日期2026-04-01 |
NCT05238961
Neuroinflammation in Asymptomatic Carotid Artery Disease - Imaging Substudy
This clinical imaging substudy will use the small molecule translocator protein (TSPO) ligand, Fludeoxyglucose(18F)-labeled DPA-714, to compare neuroinflammation in individuals with high or low grade asymptomatic carotid artery stenosis (aCAD) who are participating in the separate Neuroinflammation in Asymptomatic Carotid Artery Disease study lead by Dr. Ron Lazar (IRB-300007806). The positron emission tomography (PET) tracer [18F]DPA-714 binds to the 18 kDa translocator protein (TSPO, also known as the peripheral benzodiazepine receptor) in the mitochondria of activated microglia/macrophages and provides a non-invasive measure of neuroinflammation.
开始日期2026-04-01 |
NCT06850324
A Proof of Concept Study to Evaluate the Precision of ABL-101 Perfluorocarbon and Fluorine-19 Magnetic Resonance Imaging in Mapping of Atherosclerotic Plaque Composition and Inflammation in Patients Undergoing Carotid Endarterectomy
This study is aimed at patients suffering from carotid atherosclerosis. In the presence of atherosclerosis of the carotid arteries, one of the treatments that can be proposed is carotid endarterectomy, the aim of which is to remove the atherosclerotic plaque that obstructs the carotid artery and therefore increases the risk of developing a stroke. The risk of rupture of these plaques is primarily assessed by anatomical medical imaging: the degree of stenosis (narrowing) that the plaque causes most often informs the decision whether or not to proceed with surgery. However, it is well established that the degree of stenosis is not a very precise decision criterion: some plaques would never have ruptured, while others have ruptured even though no surgical intervention had been performed. It has since been discovered that the degree of inflammation is a much more accurate predictor of future rupture, but there is currently no reliable non-invasive imaging marker to measure plaque inflammation.
In this study, the investigators therefore evaluate a new marker, the perfluorocarbon ABL-101, for non-invasive imaging of inflammation in atherosclerotic plaque. To assess the marker's effectiveness in quantifying inflammation in plaque, the plaque will be analyzed microscopically after removal to obtain a more accurate measure of the degree of inflammation. This will enable us to assess the effectiveness of the non-invasive marker injection method versus the more invasive microscopic analysis of the removed plaque.
ABL-101 consists of a perfluorocarbon (PFC) emulsion, a liquid mixture in which PFC particles are dispersed. PFCs are chemical compounds containing only carbon and fluorine, and are known for their ability to transport large quantities of oxygen. Totally inert, PFCs cannot be broken down by the body and are eliminated naturally. Due to their small size, these particles are also captured by certain immune system cells and, combined with 19F-MRI, constitute a marker of inflammation.
In this study, the investigators therefore evaluate a new marker, the perfluorocarbon ABL-101, for non-invasive imaging of inflammation in atherosclerotic plaque. To assess the marker's effectiveness in quantifying inflammation in plaque, the plaque will be analyzed microscopically after removal to obtain a more accurate measure of the degree of inflammation. This will enable us to assess the effectiveness of the non-invasive marker injection method versus the more invasive microscopic analysis of the removed plaque.
ABL-101 consists of a perfluorocarbon (PFC) emulsion, a liquid mixture in which PFC particles are dispersed. PFCs are chemical compounds containing only carbon and fluorine, and are known for their ability to transport large quantities of oxygen. Totally inert, PFCs cannot be broken down by the body and are eliminated naturally. Due to their small size, these particles are also captured by certain immune system cells and, combined with 19F-MRI, constitute a marker of inflammation.
开始日期2025-07-01 |
100 项与 颈动脉疾病 相关的临床结果
登录后查看更多信息
100 项与 颈动脉疾病 相关的转化医学
登录后查看更多信息
0 项与 颈动脉疾病 相关的专利(医药)
登录后查看更多信息
43,170
项与 颈动脉疾病 相关的文献(医药)2025-12-31·Annals of Medicine
Prevalence of extracranial carotid artery disease in symptomatic peripheral artery disease and implications for long-term outcome
Article
作者: Staudacher, Moritz ; Zierfuss, Bernhard ; Höbaus, Clemens ; Gschwandtner, Michael ; Ceschi a Santa Croce, Anna ; Pesau, Gerfried ; Witt-Dörring, Ladislaja ; Schlager, Oliver ; Schernthaner, Gerit-Holger ; Hannes, Antonia
2025-12-31·Renal Failure
Elevated concentrations of cardiac troponin T are associated with thoracic aortic calcification in non-dialysis chronic kidney disease patients of stage G3 to G5
Article
作者: Xue, Miaorong ; Feng, Shaozhen ; Zhu, Wenjiao ; Chen, Wei ; Huang, Fengxian ; Guo, Qunying ; Lai, Zhiman ; Pan, Xiantian ; Feng, Pinning ; Chen, Xionghui ; Ke, Xiaojie ; Li, Shurong ; Xu, Yuanwen ; Mao, Haiping ; Yang, Xiao ; Li, Zhijian
2025-12-31·Annals of Medicine
Clinical features and endovascular treatment of ruptured peripheral cerebral aneurysms associated with moyamoya disease: an 8-year single-center experience
Article
作者: Fu, Chao ; Jin, Xingyi ; Chang, Yongquan ; Feng, Zheng ; Yu, Weidong
215
项与 颈动脉疾病 相关的新闻(医药)2025-04-30
Presentations include new data from BROADWAY and TANDEM Phase 3 clinical trials, demonstrating obicetrapib’s impact on key lipid and lipoprotein biomarkersNAARDEN, the Netherlands and MIAMI, April 30, 2025 (GLOBE NEWSWIRE) -- NewAmsterdam Pharma Company N.V. (Nasdaq: NAMS or “NewAmsterdam” or the “Company”), a late-stage, clinical biopharmaceutical company developing oral, non-statin medicines for patients at risk of cardiovascular disease (“CVD”) with elevated low-density lipoprotein cholesterol (“LDL-C”), for whom existing therapies are not sufficiently effective or well-tolerated, today announced it will present new clinical and preclinical data highlighting the potential for obicetrapib as a novel, oral, low-dose therapy for hypercholesterolemia, at the European Atherosclerosis Society (EAS) 93rd Congress, taking place on May 4-7, 2025 in Glasgow, UK.
Late-breaker oral presentation details are as follows:
Title: Fixed-Dose Combination of Obicetrapib and Ezetimibe for LDL-C Reduction: A Phase 3 Randomized TrialSession Name: Late Breaker Session: ClinicalOral Presentation Date and Time: Wednesday, May 5, 2025, 12:00-12:15 PM BST (7:00-7:15 AM ET)Location: William Harvey Hall
Title: Safety and Efficacy of Obicetrapib in Patients at High Cardiovascular RiskSession Name: Late Breaker Session: ClinicalOral Presentation Date and Time: Wednesday, May 5, 2025, 12:15-12:30 PM BST (7:15-7:30 AM ET)Location: Will Harvey Hall
Additional session details are as follows:
Title: Obicetrapib Reduces Atherosclerosis and Vascular Inflammation, Mainly by Reducing Non-HDL Cholesterol, Improves Lesion Stability and Adds to the Beneficial Effects of EzetimibeSession Name: SaaG Session: Emerging Lipid-Lowering Strategies: Mechanisms, Pre-clinical Studies and OutcomesPresentation Date and Time: Monday, May 5, 2025, 1:58-2:05 PM BST (8:58-9:05 AM ET)Location: Station 7 Title: Obicetrapib Alone and in Combination with Ezetimibe Increases Reverse Cholesterol Transport and Does Not Affect VLDL ProductionSession Name: SaaG Session: Emerging Lipid-Lowering Strategies: Mechanisms, Pre-clinical Studies and OutcomesPresentation Date and Time: Monday, May 5, 2025, 2:05-2:12 PM BST (9:05-9:12 AM ET)Location: Station 7
Title: Low-Dose Obicetrapib Significantly Increases Concentrations of Lipophilic Antioxidants, ApoE, and S1P in HDL SubfractionsSession Name: SaaG Session: Lipid Lowering Therapies and Advances in Vascular TherapeuticsPresentation Date and Time: Monday, May 5, 2025, 2:19-2:26 PM BST (9:19-9:26 AM ET)Location: Station 4
Title: Obicetrapib Significantly Increases Plasma and High-Density Lipoprotein (HDL) Levels of Lipophilic AntioxidantsSession Name: SaaG Session: Lipoprotein Dynamics: HDL to RemnantsPresentation Date and Time: Monday, May 5, 2025, 3:17-3:24 PM BST (10:17-10:24 AM ET)Location: Station 3
Title: Efficacy and Safety of Combination Obicetrapib with Moderate-Dose StatinsSession Name: Poster Networking SessionsPresentation Date and Time: Monday, May 5 & Tuesday, May 6, 2025, 5:20 - 6:50 PM BST (12:17-1:24 PM ET)Location: Poster Board #246, Exhibition Hall Title: Safety and Efficacy of Cholesteryl Ester Transfer Protein Inhibition: From Genetics to Outcome TrialsSession Name: Workshop: New risk factors for atherosclerotic diseasePresentation Date and Time: Wednesday, May 7, 2025, 12:20 -12:30 PM BST (7:17-8:24 AM ET)Location: James Black Hall
About NewAmsterdam
NewAmsterdam Pharma (Nasdaq: NAMS) is a late-stage biopharmaceutical company whose mission is to improve patient care in populations with metabolic diseases where currently approved therapies have not been adequate or well tolerated. We seek to fill a significant unmet need for a safe, well-tolerated and convenient LDL-lowering therapy. In multiple phase 3 trials, NewAmsterdam is investigating obicetrapib, an oral, low-dose and once-daily CETP inhibitor, alone or as a fixed-dose combination with ezetimibe, as LDL-C lowering therapies to be used as an adjunct to statin therapy for patients at risk of CVD with elevated LDL-C, for whom existing therapies are not sufficiently effective or well tolerated.
Company Contact
Matthew PhilippeP: 1-917-882-7512matthew.philippe@newamsterdampharma.com
Media Contact
Spectrum Science on behalf of NewAmsterdamJaryd LeadyP: 1-856-803-7855jleady@spectrumscience.com
Investor Contact
Precision AQ on behalf of NewAmsterdamAustin MurtaghP: 1-212-698-8696austin.murtagh@precisionaq.com
临床3期
2025-04-29
Orchestra BioMed’s Virtue® Sirolimus AngioInfusion Balloon™ (“Virtue SAB”) is the only non-coated drug-eluting balloon system under clinical investigation worldwide and has been awarded multiple FDA Breakthrough Device DesignationsThe Virtue Trial will be the first U.S. IDE head-to-head randomized evaluation of a sirolimus-eluting balloon versus a commercially available paclitaxel-coated balloon (AGENT™) for the treatment of coronary in-stent restenosis (“ISR”)Robust non-inferiority trial is designed to provide a clear pathway to regulatory approval as well as potentially showcase clinical advantages of Virtue SABWith the amended IDE approved by the FDA, Orchestra BioMed is currently targeting initiation of the Virtue Trial during the second half of 2025 NEW HOPE, Pa., April 29, 2025 (GLOBE NEWSWIRE) -- Orchestra BioMed Holdings, Inc. (Nasdaq: OBIO, “Orchestra BioMed” or the “Company”), a biomedical company accelerating high-impact technologies to patients through risk-reward sharing partnerships, today announced that the U.S. Food and Drug Administration (“FDA”) has approved its Investigational Device Exemption (“IDE”) amendment to initiate an updated design of the Company’s planned Virtue SAB in the Treatment of Coronary ISR Trial (“Virtue Trial”). The IDE provides FDA regulatory clearance for Orchestra BioMed to initiate a U.S. pivotal clinical trial comparing its highly differentiated, next-generation Sirolimus-AngioInfusion Balloon, Virtue SAB to the Boston Scientific AGENT paclitaxel-coated balloon, currently the only drug-coated balloon (“DCB”) FDA-approved for a coronary indication. Data from the Virtue Trial will be used to support regulatory approval in the U.S. Virtue SAB and SirolimusEFR are investigational technologies owned by Orchestra BioMed, which also controls and is responsible for all regulatory filings, clinical operations, and drug and device supplies for the Virtue Trial. The Differentiation of Virtue SAB, An FDA Breakthrough Designated DeviceVirtue SAB is designed to deliver a proprietary extended-release formulation of sirolimus, SirolimusEFR™, through a non-coated microporous AngioInfusion™ Balloon that protects the drug in transit to consistently deliver a large liquid dose, overcoming certain limitations of DCBs. SirolimusEFR™ enables tissue uptake and extended release of the required therapeutic levels of sirolimus (> 1ng/mg tissue concentration), the “gold-standard” drug for preventing arterial restenosis, through the critical healing period of approximately 30 days. Virtue SAB has been previously granted FDA Breakthrough Device Designation for the treatment of coronary ISR as well as for coronary small vessel disease and peripheral artery disease below-the-knee. In the multi-center SABRE pilot study, Virtue SAB demonstrated best-in-class clinical results for the treatment of coronary ISR, including 12-month target lesion failure of 2.8% and 6-month late lumen loss of 0.12mm. “Virtue SAB has the potential to be one of the most compelling technologies in interventional cardiology. It’s the only product in development that optimizes both the arterial tissue uptake and retention of sirolimus to achieve pharmacokinetics that match or even exceed those of proven ‘limus-eluting stents,” said Dean J. Kereiakes, M.D., FACC, MSCAI, Chairman of The Christ Hospital Heart & Vascular Institute, Medical Director of The Christ Hospital Research Institute, Professor of Clinical Medicine at The Ohio State University, Professor of Medicine at University of Cincinnati and Co-Principal Investigator on the Virtue Trial. Dr. Kereiakes continued: “Virtue SAB is designed to consistently deliver a large liquid dose of an extended-release formulation of sirolimus to overcome certain limitations of traditional DCBs, including lower doses due to surface area and coating integrity constraints, drug loss in transit leading to inconsistent dosing, and the risk of emboli from large coating particulates. As the coronary treatment landscape continues to shift toward more rapid adoption of DCBs, I’m excited about the potential of Virtue SAB to set a new standard of care.” “Drug-coated balloons are emerging as a new standard of care in the treatment of various coronary and peripheral indications, and I believe utilization of this class of technology will continue to grow and evolve over time as the science is expanding. In a field largely reliant on paclitaxel drug-coated balloons, Virtue SAB stands out as the only device with a completely different mechanism of action; namely to provide delivery of a large liquid dose of an extended-release formulation sirolimus," commented Allen Jeremias, MD, MSc, Associate Director of the Cardiac Catheterization Laboratory at St. Francis Hospital & Heart Center, and Co-Principal Investigator on the Virtue Trial. “Having had the opportunity to work with several DCBs, I anticipate continued momentum for this class, and am eager to see how Virtue SAB, particularly with its anti-restenotic, anti-inflammatory and cytostatic SirolimusEFR formulation, performs in a head-to-head trial against a paclitaxel-coated balloon.” Showcasing Distinctive and Sustainable Advantages of Virtue SAB Through a Head-to-Head Trial “We believe there is a multibillion-dollar U.S. market for coronary drug delivery balloons based on the significant unmet clinical need, market demand, and established reimbursement,” said David Hochman, Chairman and Chief Executive Officer of Orchestra BioMed. “We made a deliberate, strategic decision to pursue a head-to-head trial with the commercially available AGENT paclitaxel-coated balloon, underscoring our confidence in Virtue SAB as a fundamentally differentiated solution for the treatment of atherosclerosis. We believe this approach offers the most direct path to regulatory approval while also providing the best opportunity to demonstrate what we believe are distinctive and sustainable advantages of our proprietary technology.” Mr. Hochman continued, “The superior safety and efficacy of sirolimus over paclitaxel was made clear by the performance of drug-eluting stents. Published meta-analysis involving 76 trials show ‘limus-eluting stents have significantly better clinical performance than paclitaxel-eluting stents in terms of target lesion revascularization and major adverse cardiac events.1 We believe this is due to their ability to maintain sufficient drug tissue concentration through the critical healing period of approximately 30 days, because stents that failed to do this did not perform well clinically.2,3,4 Virtue SAB is the only drug delivery balloon that has demonstrated comparable drug tissue levels to clinically successful drug-eluting stents in large published preclinical studies, without the need to leave a permanent metal implant in the artery. Our pilot clinical results with Virtue SAB also highlight the potential for optimal clinical outcomes with robust sirolimus delivery. We’re excited to showcase the full potential of Virtue SAB in this landmark trial and are proud of our team and grateful to our clinical collaborators for their work in achieving this important milestone.” The Virtue Trial is a prospective, multi-center, randomized trial comparing clinical outcomes of Virtue SAB to AGENT Paclitaxel DCB in the treatment of coronary ISR, a difficult-to-treat and serious complication of coronary stenting. The primary endpoint is a non-inferiority comparison of Target Lesion Failure (TLF) defined as a composite of cardiac death, nonfatal target vessel myocardial infarction and ischemia-driven target lesion revascularization at 12 months. The trial will randomize 740 patients across up to 75 centers in the U.S. With the amended IDE approved by the FDA, Orchestra BioMed is currently targeting initiation of the Virtue Trial during the second half of 2025, bringing the Company one step closer to delivering a next-generation solution for atherosclerotic disease. About Coronary In-Stent Restenosis (ISR)Coronary ISR is a serious complication of coronary stenting, which can increase the risk of life-threatening heart problems. It is characterized by a re-narrowing of a coronary artery segment that was previously treated with a stent. According to the National Cardiovascular Data Registry, coronary ISR occurs in up to 10% of stented patients during the first year and continues at a rate of up to 3% per year thereafter, resulting in an estimated over 325,000 coronary ISR lesions annually worldwide that may require treatment. If left untreated, coronary ISR may lead to stable angina, unstable angina, acute coronary syndrome, acute myocardial infarction, or death. About Virtue SABVirtue SAB is designed to deliver a proprietary extended-release formulation of sirolimus, SirolimusEFR™ through a non-coated microporous AngioInfusion™ Balloon that protects the drug in transit to consistently deliver a large liquid dose overcoming certain limitations of drug-coated balloons. SirolimusEFR delivered by Virtue SAB has been shown in published preclinical series involving hundreds of arterial deliveries to achieve sustained tissue levels well above the known required therapeutic tissue concentration for inhibiting restenosis (1 ng/mg tissue) for the entire critical healing period of approximately 30 days. Virtue SAB demonstrated positive three-year clinical data in coronary ISR in the SABRE study, a multi-center prospective, independent core lab-adjudicated clinical study of 50 patients conducted in Europe. Virtue SAB has been granted Breakthrough Device Designation by the FDA for specific indications relating to coronary ISR, coronary small vessel disease and peripheral artery disease below-the-knee. About Orchestra BioMed Orchestra BioMed (Nasdaq: OBIO) is a biomedical innovation company accelerating high-impact technologies to patients through risk-reward sharing partnerships with leading medical device companies. Orchestra BioMed’s partnership-enabled business model focuses on forging strategic collaborations with leading medical device companies to drive successful global commercialization of products it develops. Orchestra BioMed’s lead product candidate is atrioventricular interval modulation (AVIM) therapy for the treatment of hypertension, the leading risk factor for death worldwide. Orchestra BioMed is also developing the Virtue® Sirolimus AngioInfusion™ Balloon (SAB) for the treatment of atherosclerotic artery disease, the leading cause of mortality worldwide. Orchestra BioMed has a strategic collaboration with Medtronic, one of the largest medical device companies in the world, for development and commercialization of AVIM therapy for the treatment of hypertension in pacemaker-indicated patients, and a strategic partnership with Terumo, a global leader in medical technology, for development and commercialization of Virtue SAB for the treatment of artery disease. The company has received four Breakthrough Device Designations from the U.S. FDA across these two core programs, reflecting the significant potential of its technologies to address high unmet needs in cardiovascular care. For further information about Orchestra BioMed, please visit www.orchestrabiomed.com, and follow us on LinkedIn. References to Websites and Social Media Platforms References to information included on, or accessible through, websites and social media platforms do not constitute incorporation by reference of the information contained at or available through such websites or social media platforms, and you should not consider such information to be part of this press release. Forward-Looking StatementsCertain statements included in this press release that are not historical facts are forward-looking statements for purposes of the safe harbor provisions under the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements generally are accompanied by words such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “should,” “would,” “plan,” “predict,” “potential,” “seem,” “seek,” “future,” “outlook” and similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These forward-looking statements include, but are not limited to, statements relating to the timing of the initiation and of the Virtue Trial, the number of patients to be enrolled in the Virtue Trial, and the potential safety and efficacy of the Company’s product candidates, including the ability of Virtue SAB to overcome certain limitations of DCBs. These statements are based on various assumptions, whether or not identified in this press release, and on the current expectations of the Company’s management and are not predictions of actual performance. These forward-looking statements are provided for illustrative purposes only and are not intended to serve as and must not be relied on as a guarantee, an assurance, a prediction, or a definitive statement of fact or probability. Actual events and circumstances are difficult or impossible to predict and may differ from assumptions. Many actual events and circumstances are beyond the control of the Company. These forward-looking statements are subject to a number of risks and uncertainties, including changes in domestic and foreign business, market, financial, political, and legal conditions; risks related to regulatory approval of the Company’s product candidates; the timing of, and the Company’s ability to achieve, expected regulatory and business milestones; the impact of competitive products and product candidates; and the risk factors discussed under the heading “Item 1A. Risk Factors” in the Company’s annual report on Form 10-K filed with the U.S. Securities and Exchange Commission on March 31, 2025, as updated by any risk factors disclosed under the heading “Item 1A. Risk Factors” in the Company’s subsequently filed quarterly reports on Form 10-Q. The Company operates in a very competitive and rapidly changing environment. New risks emerge from time to time. Given these risks and uncertainties, the Company cautions against placing undue reliance on these forward-looking statements, which only speak as of the date of this press release. The Company does not plan and undertakes no obligation to update any of the forward-looking statements made herein, except as required by law. Investor ContactJeremy FefferLifeSci AdvisorsJfeffer@lifesciadvisors.com Media ContactKelsey Kirk-EllisOrchestra BioMedkkirkellis@orchestrabiomed.com References 1Xinlin Zhang, et. al. PLOS ONE 2014 May 20;9(5):e97934. 2https://slideplayer.com/slide/5787004. 3 Tada, et. Al., Am Heart J. 2013 Jan;165(1):80-6. 4Leon M LBT III, Session 3014 ACC 2011.
临床结果临床研究突破性疗法
2025-04-29
·动脉网
近期,关税政策成为世界关注的焦点。多轮加征后,美国对中国的关税比例提高至145%。国务院关税税则委员会则在4月11日发布公告称,对原产于美国的进口商品加征关税税率提高至125%。临床刚需属性与高端技术壁垒的双重叠加,使得医疗产品关税波动成为中美战略竞争的焦点领域。当前中美战略竞争持续深化,医疗领域自主可控与供应链安全已成为国家战略核心议题。只有掌握自主权,才拥有更大的话语权。根据中信建投数据,2023年,神经介入和外周介入领域美国企业合计在中国市场份额超过65%。根据IQVIA数据,神经介入、外周介入市场规模分别超过百亿,也就是说两大市场超过一半的手术依赖美国产品供应。在关税政策调整的背景下,临床用量大且进口依赖度高的产品往往首当其冲。然而,在国产替代浪潮的持续推进中,进口依赖度仍较高的产品,通常也是国产化难度较大、对产品性能和质量要求较高的产品。但另一面在关税政策变化契机下,国产也迎来啃下国产替代硬骨头的契机。面对这一机遇,中天医疗凭借完备的产品线、创新的产品设计和可靠的产品品质,有望在这一窗口期,成为国产替代主力军。01全球首创 工艺升维中天医疗天织™编织型微导丝在神经介入手术中,已经有医生开始担心神经介入微导丝的供应。微导丝是神经介入手术成败的关键器械,由于颅内血管相对迂曲和脆弱,对微导丝的安全和性能都提出了更高的要求,微导丝既要有足够的柔顺性,又需要良好的操控性,同时还需要有足够的支撑性,指引后续器械的通过。由于临床对产品性能的高要求,国内神经介入微导丝长期依赖进口品牌供应。根据公众号“医休神介说”数据,某进口品牌每年在国内的出货量超过30万条。然而,当前复杂的国际局势给其供应链稳定性带来了严峻挑战。高性能微导丝的研发技术壁垒极高。某进口品牌S系列其核心壁垒之一是海波管切割工艺。这种工艺在保证微导丝柔顺性的同时,还能实现1:1扭控,让医生能够精准操控导丝。此外,其专利布局覆盖了核心设计,进一步巩固了其市场地位。然而,海波管工艺也存在改进空间。导丝过度旋转时有断裂风险,一旦发生,将带来严重的医疗事故风险。在神经介入微导丝中,中天医疗的天织™编织型微导丝独辟蹊径,经过五年研发,该产品成为全球首款编织型微导丝。与传统产品相比,天织™编织型微导丝不仅可以实现1:1扭控和更出色的塑形保持能力,其性能足以比肩进口导丝。更重要的是,由于其独特的结构设计原理,该产品能够完全规避导丝断裂风险。中天天织™编织型微导丝不仅能够实现国产替代,还带来了性能的显著提升。同时,该产品拥有完全独立自主的知识产权,有望成为真正实现国产替代的神经微导丝,为国内神经介入手术提供更安全、更可靠的器械选择。中天医疗天织™微导丝与某进口品牌S导丝特点高性能微导丝作为神经介入手术通路类器械,临床需求的快速增长反映出神经介入市场强劲的需求,对企业产品迭代也提出更高要求。能够在竞争激烈的市场中补齐国产关键短板,中天医疗的差异化优势是丰富的产品解决方案+领先的创新术式,深度满足临床需求,引领行业发展。02血栓清除,国产优选中天医疗外周血栓清除SWAM创新解决方案在外周介入市场,同样也有关键产品依赖进口供应。外周血栓抽吸系统同样是美国企业主导的市场,临床亟待安全、有效的血栓清除解决方案。据弗若斯特沙利文数据,中国治疗深静脉血栓的血栓抽吸手术数量预计在2030年将达到52.8万例,2024年至2030年的复合年增长率为24.4%。目前,国内血栓抽吸市场主要由Straub和AnjioJet这两大产品主导,市场份额超过50%。该领域技术壁垒较高,血栓抽吸系统需要在抽吸导管的通过性、抗打折性、抽吸效率以及降低失血量等方面实现平衡。在这一高增长的术式中,中天医疗推出外周血栓清除SWAM创新解决方案(天航®抽吸导管+天戟外周栓塞移除装置)。SWAM融合了机械血栓抽吸和物理抽吸的优势,其核心组件分离器是该系统的一大特色,中天天戟外周栓塞移除装置能够高效切割并清除阻塞血管的血栓,尤其在处理大负荷量血栓时,可显著提高抽吸效率。此外,天航®抽吸导管的设计也颇具亮点。导管头端具备不同的抽吸角度,能更有效地清除附壁血栓。导管采用多段式结构设计,由不同硬度的多节段材料(如PEBAX远端+刚性近端)组成,在保证灵活性的同时提升了支撑力,便于通过迂曲血管或狭窄病变段,并有效防止抽吸时管腔塌陷,确保抽吸过程的稳定性和效率。03完全可控,领先市场中天医疗混天绫®外周可解脱带纤维毛弹簧圈栓塞系统在外周介入领域,带纤毛可控弹簧圈目前也仍然高度依赖进口供应。弹簧圈用于血管栓塞,在外周血管介入领域应用广泛,包括:腹主动脉瘤腔内治疗的内漏管理、内脏动脉瘤的栓塞治疗、咯血、动静脉瘘等术式。国内外周介入市场带纤维毛的可控弹簧圈目前仍以进口品牌为主,进口品牌的半可控产品主导市场。然而,进口产品也存在不足,其半可控技术存在术中意外解脱的风险。单一产品主导市场不仅带来断供风险,而且临床医生也需要一款完全可控的外周弹簧圈。在国产外周带纤毛可控弹簧圈中,中天医疗的混天绫®外周弹簧圈脱颖而出,成为国产替代的优先选择。混天绫®外周弹簧圈能够实现完全可控精准栓塞,可多次回拉调整进行精准定位,防止意外解脱,从而大大提升精准栓塞效果。中天医疗的混天绫®外周可解脱带纤维毛弹簧圈栓塞系统通过三大核心技术突破,实现了精准栓塞与安全操控。一是可控精准栓塞创新的机械解脱设计,采用自膨钳锁球头,支持一键解锁回撤功能。即使弹簧圈推出导管后,仍可调整或重构,有效防止术中意外解脱。二是带纤毛设计,更容易成栓,且纤维毛纤细顺滑,显著降低输送阻力。三是自研高真空热处理工艺,确保弹簧圈的柔软度,提升操作的灵活性和安全性。混天绫®外周弹簧圈已经在全国多家三甲医院完成了数千例临床应用,并获得了广泛认可,有望加速其在临床的大规模应用。外周介入集采频繁,市场竞争激烈,只有具备竞争力的产品才能在外周介入市场立足。国内企业需在产品升级上做“加法”,同时在布局上做“减法”,聚焦临床需求大、增长快的市场。中天医疗前瞻性地布局了外周介入领域多个高技术壁垒、大临床需求的产品,产品覆盖外周动静脉多种疾病。并且凭借领先的产品进度,后发先至,迅速在外周介入市场占据优势。 04国产空白,创新领航中天医疗天宓®颈动脉支架目前,血管介入领域国产化剩下的都是难啃的硬骨头,对国产产品的差异化和品质要求都很高。以劲动脉支架为例,这一市场目前还处于国产空白。在医疗器械国产化进程中,尽管多数品类已实现替代或部分替代,但颈动脉支架领域仍面临“卡脖子”困境——国内尚无国产产品获批上市。颈动脉狭窄是引起缺血性卒中的主要原因之一。颈动脉支架需求量较大,据采访了解,国内颈动脉支架年手术量超过15万台。进口第一代颈动脉支架目前是国内临床应用较多的产品,这款产品网孔较大,术后斑块脱落风险高,易引发小卒中或静止性卒中,严重威胁患者预后。进口第二代产品新近获批,虽然在产品上有所优化,但也存在不足。第二代颈动脉支架设计理念是缩小颈动脉支架的网孔,从而减少术后卒中的发生率。进口二代颈动脉支架采用双层支架缩小网孔,这一技术路径难以避免的问题是需要采用直径较大的输送导管,增加了术中对血管的刺激。同时,进口二代颈动脉支架普遍采用激光切割工艺,在弯曲血管处释放后,血管内壁有支架杆突出,进而容易造成血栓。第三代颈动脉支架的代表是中天医疗天宓®颈动脉支架,这一产品已进入创新医疗器械“绿色通道”。三代颈动脉支架产品特点在缩小颈动脉支架网孔方面,天宓®颈动脉支架采用了全球首创的单层微网混合编织技术,网孔面积仅为传统切割型支架的1/30,能阻拦斑块,显著降低斑块脱垂风险。同时不影响输送性能,能够通过5F超细输送系统(兼容6F导管),实现经桡/经股多路径灵活操作,提升手术安全性。在工艺上中天医疗采用精密编织工艺,解决了激光雕刻支架在血管弯曲处对血管的刺激。这款产品不仅有望实现进口替代,也将为临床带来革新性的产品,降低颈动脉支架断供风险。在中美战略竞争格局下,医疗科技领域的战略制高点争夺已成为衡量国家综合实力的关键指标。从神经介入到外周介入再到颈动脉支架,中天医疗能够在多个关键产品实现前瞻式布局,基于临床洞察进行差异化创新,而非一味跟随,这背后源于其三位一体的核心竞争力:技术护城河(掌握底层技术平台与自主知识产权)、创新源动力(突破“卡脖子”技术的原始创新能力)、价值转化力(创新产品快速商业化的能力)。技术创新,大有可为。中天医疗作为血管介入领域先锋企业,除了产品实力过硬,商业化表现也非常亮眼,营收已实现破亿。破解中天医疗从技术突破到产业主导的关键能力,不难发现,中天医疗抓住了多个增长机遇。在多个集采中中标,撬动产品大规模入院和商业化,凭借良好的临床应用口碑,推动产品持续增长,形成可持续发展模式。通过技术壁垒构建-集采规模效应-临床口碑反哺的正向循环,实现持续增长,也才能在国产替代的关键窗口期厚积薄发。如果您想对接文章中提到的项目,或您的项目想被动脉网报道,或者发布融资新闻,请与我们联系;也可加入动脉网行业社群,结交更多志同道合的好友。近期推荐声明:动脉网所刊载内容之知识产权为动脉网及相关权利人专属所有或持有。未经许可,禁止进行转载、摘编、复制及建立镜像等任何使用。动脉网,未来医疗服务平台
分析
对领域进行一次全面的分析。
登录
或
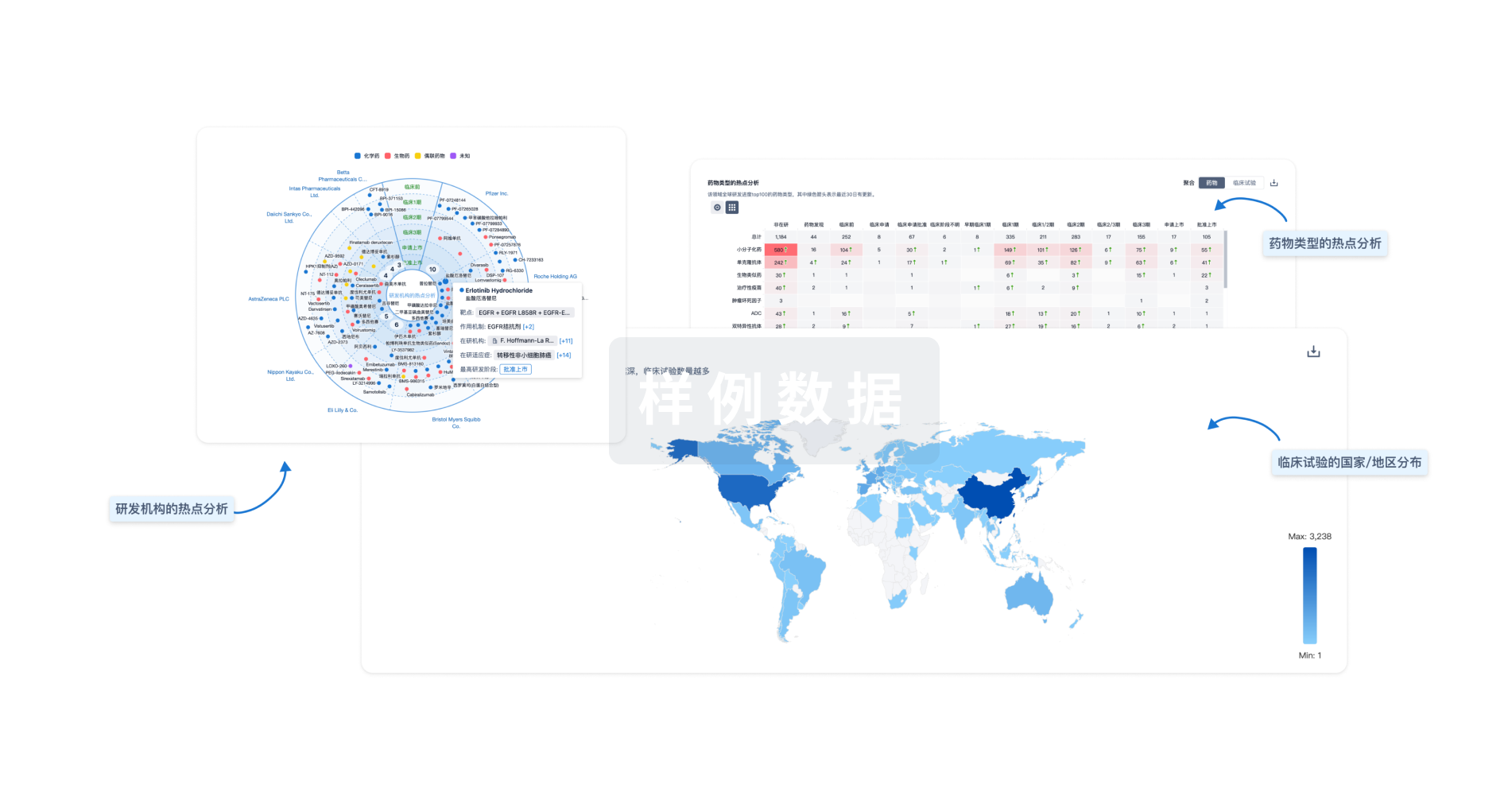
生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用






