更新于:2024-11-01
Teva Pharmaceuticals USA, Inc.
更新于:2024-11-01
概览
标签
其他疾病
神经系统疾病
内分泌与代谢疾病
小分子化药
单克隆抗体
生物类似药
疾病领域得分
一眼洞穿机构专注的疾病领域
暂无数据
技术平台
公司药物应用最多的技术
暂无数据
靶点
公司最常开发的靶点
暂无数据
| 排名前五的药物类型 | 数量 |
|---|---|
| 小分子化药 | 10 |
| 单克隆抗体 | 4 |
| 生物类似药 | 2 |
关联
48
项与 Teva Pharmaceuticals USA, Inc. 相关的药物靶点 |
作用机制 CGRP拮抗剂 |
在研适应症 |
最高研发阶段批准上市 |
首次获批国家/地区 美国 |
首次获批日期2018-09-14 |
作用机制 NET抑制剂 [+1] |
在研机构 |
在研适应症 |
非在研适应症- |
最高研发阶段批准上市 |
首次获批国家/地区 美国 |
首次获批日期2013-10-11 |
233
项与 Teva Pharmaceuticals USA, Inc. 相关的临床试验A Single Center, Randomized, Double Blind, Placebo-Controlled, Parallel Group Study to Evaluate the Efficacy and Safety of Fremanezumab (675 mg Quarterly) in Female Patients Aged 18-45 With Menstrual Migraine
The goal of this clinical trial is to learn about how a migraine prevention medicine works for people who have migraines/headaches with their menstrual period. The study includes people ages 18 to 45 who have been diagnosed with migraine and who have a migraine with their menstrual period or those who have migraines with their menstrual period and at other times of the month as well.
The main question the study aims to answer are:
• Does fremanazemab, an injectable calcitonin gene-related peptide (CGRP) pathway targeting therapy, decrease migraines associated with menstruation?
Participants will
have an evaluation and examination by a headache specialist physician
will receive the study medicine or inactive substitute every three months for two treatments
fill out diaries about their migraines
have tests on saliva to measure hormone levels
Researchers will compare the people who get the medicine to those who get the inactive substitute to see if there are differences in response.
The main question the study aims to answer are:
• Does fremanazemab, an injectable calcitonin gene-related peptide (CGRP) pathway targeting therapy, decrease migraines associated with menstruation?
Participants will
have an evaluation and examination by a headache specialist physician
will receive the study medicine or inactive substitute every three months for two treatments
fill out diaries about their migraines
have tests on saliva to measure hormone levels
Researchers will compare the people who get the medicine to those who get the inactive substitute to see if there are differences in response.
开始日期2024-03-01 |
申办/合作机构 |
Expertise Asthma COPD Program With Digital Support
The aim EXACT@Home is to create an evidence-based health program using e.g. questionnaires, a digital health platform and multiple digital devices to further improve the assessment of patients diagnosed with severe asthma. By better charting treatable traits (e.g. poor adherence, physical inactivity, dysfunctional breathing), we expect to improve the indication for the use of biologics. One the devices that will be used is also a medicinal product: a digital inhaler, which monitors adherence and inhaler technique through its connected application and aims to improve adherence and inhaler technique with reminders and notifications. Next to this an activity tracker, hand-held spirometer and FeNO measuring device will be used. The information of the devices will be collected in a Personal Digital Healthcare Environment (PDHE). Patients diagnosed with severe asthma according to the regional asthma Multi-Disciplinary Team Meeting (MDTM) eligible for a treatment with biologics will be included. Half of the patients will immediately receive a biologic. The other half will first undergo the systematic assessment including home monitoring (=EXACT@home) and afterwards a treatment will be chosen based on this evaluation: optimization of treatable traits when present and/or biologics. The chosen treatment of both, the intervention and control group, will be evaluated during 11-12 months.
开始日期2023-01-31 |
申办/合作机构 |
A Randomized, Double-Blind, Placebo-Controlled, Parallel- Design, Multiple-Site Study to Evaluate the Therapeutic Equivalence of Estradiol Vaginal Inserts 4 mcg (Teva Pharmaceuticals, Inc.) With IMVEXXY® (Estradiol Vaginal Inserts) (TherapeuticsMD, Inc.) in the Treatment of Dyspareunia in Women With Vulvar and Vaginal Atrophy
Randomized, double-blind, placebo-controlled, parallel-designed, multiple-site, bioequivalence study with clinical endpoints.
开始日期2022-11-15 |
100 项与 Teva Pharmaceuticals USA, Inc. 相关的临床结果
登录后查看更多信息
0 项与 Teva Pharmaceuticals USA, Inc. 相关的专利(医药)
登录后查看更多信息
10
项与 Teva Pharmaceuticals USA, Inc. 相关的文献(医药)2019-01-01·Journal of Biotechnology3区 · 工程技术
An enhanced regeneration strategy to improve microbial control and prolong resin lifetime for Protein A resin in large-scale monoclonal antibody (mAb) purification
3区 · 工程技术
Article
作者: Zhou, Tianyi ; Zhang, Zhaoqing ; Wang, Lu ; Jin, Mi
2018-07-01·Therapeutic Innovation & Regulatory Science
Response to “Use of Qualitative Data to Support Content Validity of Performance-Based Cognitive Outcome Assessments”
Letter
作者: Gordon, Mark Forrest ; Hannesdottir, Kristin ; Ropacki, Michael T ; Hendrix, Suzanne ; Stern, Robert A ; Stephenson, Diane ; Coons, Stephen Joel
2016-04-01·AAPS PharmSciTech3区 · 医学
A Quality by Experimental Design Approach to Assess the Effect of Formulation and Process Variables on the Extrusion and Spheronization of Drug-Loaded Pellets Containing Polyplasdone® XL-10
3区 · 医学
Article
作者: Mallipeddi, Rama ; Neau, Steven H ; Loka, Nikhil C ; Rane, Anuja M ; Saripella, Kalyan K
39
项与 Teva Pharmaceuticals USA, Inc. 相关的新闻(医药)2024-10-17
Company Grants Teva a License to Market Generic Galafold® Beginning in January 2037PRINCETON, N.J., Oct. 17, 2024 (GLOBE NEWSWIRE) -- Amicus Therapeutics (Nasdaq: FOLD) today announced that it has entered into a License Agreement (Agreement) with Teva Pharmaceuticals USA, Inc. and Teva Pharmaceuticals, Inc. (collectively Teva). This Agreement resolves the patent litigation brought by Amicus in response to Teva’s Abbreviated New Drug Application (ANDA) seeking approval to market a generic version of GALAFOLD® (migalastat) 123mg capsules prior to expiration of the applicable patents. Pursuant to the terms of the Agreement, Amicus will grant Teva a license to market its generic version of GALAFOLD® in the United States beginning on January 30, 2037, if approved by the U.S. Food and Drug Administration (FDA) and unless certain limited circumstances customarily included in these types of agreements occur. In accordance with the Agreement, the parties will terminate all ongoing Hatch-Waxman litigation between Amicus and Teva regarding GALAFOLD® patents pending in the U.S. District Court for the District of Delaware. The litigation will continue against Aurobindo1 as the remaining active party and the litigation stay remains in place for Lupin2. As required by law, the companies will submit the confidential license agreement to the U.S. Federal Trade Commission and the U.S. Department of Justice for review. About Amicus Therapeutics Amicus Therapeutics (Nasdaq: FOLD) is a global, patient-dedicated biotechnology company focused on discovering, developing and delivering novel high-quality medicines for people living with rare diseases. With extraordinary patient focus, Amicus Therapeutics is committed to advancing and expanding a pipeline of cutting-edge, first- or best-in-class medicines for rare diseases. For more information please visit the company’s website at www.amicusrx.com, and follow on X and LinkedIn. Forward Looking StatementThis press release contains "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995, including but not limited to, statements concerning: the terms of the settlement agreement with Teva, expectations regarding the impact of the settlement agreement and submission of the settlement agreement for review to the United States Federal Trade Commission and the United States Department of Justice. The company cautions that forward-looking statements are inherently uncertain. Although the company believes that such statements are based on reasonable assumptions within the bounds of its knowledge of its business and operations, the forward-looking statements are neither promises nor guarantees and they are necessarily subject to a high degree of uncertainty and risk. Actual performance and results may differ materially from those expressed or implied in the forward-looking statements due to various risks and uncertainties, including the unfavorable outcome of other litigation, including so-called "Paragraph IV" litigation and other patent litigation, related to GALAFOLD, which may lead to competition from generic drug manufacturers; the outcome of any review of the settlement agreement by the United States Federal Trade Commission and United States Department of Justice; the U.S. FDA may approve Teva's ANDA significantly in advance of the entry date; and those risks and uncertainties described under the heading "Risk Factors" in the company's most recent Annual Report on Form 10-K for the year ended December 31, 2023, and on Form 10-Q for the quarter ended June 30, 2024. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, and we undertake no obligation to revise or update this news release to reflect events or circumstances after the date hereof. CONTACT: Investors:Amicus TherapeuticsAndrew FaughnanVice President, Investor Relationsafaughnan@amicusrx.com (609) 662-3809 Media:Amicus TherapeuticsDiana MooreHead of Global Corporate Affairs and Communicationsdmoore@amicusrx.com (609) 662-5079 FOLD–G 1 Aurobindo Pharma LTD and Aurobindo Pharma USA, Inc.2 Lupin LTD and Lupin Pharmaceuticals, Inc.
引进/卖出专利侵权
2024-10-13
有些时候,看上去打着为患者解决药品经济负担的所谓“慈善基金会”,也会成为药企垄断提升药价的工具。
4年前,美国司法部对以色列Teva制药在美国的下辖子公司Teva Pharmaceuticals USA Inc .和Teva Neuroscience Inc.发起诉讼,指控他们通过“表面上独立的基金会”非法支付其多发性硬化症(MS)产品Copaxone的医疗保险付款。在这一过程中,Teva通过一系列的合谋抬价,以超过通货膨胀率19倍的速度提高其药物Copaxone的价格,最终让药品成本上涨了329%。
如今Teva为恶意抬价和非法回扣的指控支付了4.5亿美元和解费。
事件主角Copaxone
Copaxone毫无疑问是一款1996年上市的老药,是一种人工合成的肽类制剂。由L-丙氨酸、L-赖氨酸、L-谷氨酸、L-酪氨酸四种氨基酸按一定比例(4.2:3.4:1.4:1)聚合而成的多肽混合物。这四种氨基酸在髓鞘碱性蛋白中常见,Copaxone在体内可以作为免疫系统的诱饵,从而减缓自身免疫作用,因此可以用于MS这一疾病。
自上市以来,这款药物一直是Teva的销量超级重磅炸弹,2014年销售额达到了42.4亿美元,按理来说,自2015年Copaxone基础专利期到期以后,这款药物的销量就该出现断崖式下滑了。
但是Teva显然不想要这款药物的利润从公司手心中溜走,为了确保销量的桂冠,这家公司可以说是无不用其极,从多个方面重拳出击。
重拳出击
在专利方面,Teva发起了十几项针对仿制药的专利诉讼,在基础专利到期后,该公司利用重叠专利让竞争对手陷入了旷日持久的专利战。
在法规方面,该公司试图通过美国药品监管法达成目的。因为Copaxone是一种多肽混合物,Teva试图将Copaxone认定为生物制品,被认定为生物制品那么原本所谓的仿制药就将成为生物类似物,而Copaxone作为处方的不可取代性也将更为稳固。
而这里,Teva的控价阴谋则涉及慈善基金会,药房,药商(其他竞争对手)。
从2007年到2015年间,Teva向两个慈善基金会——援助基金会(TAF)和慢性病基金会(CDF)支付款项,试图使用Teva的钱来支付服用Copaxone的患者的医疗保险共付额。在同一时期,Teva将Copaxone的价格就从每年约17000美元提高到每年73000美元以上。
不过这只是提价阴谋的一部分,药房供应商Advanced Care Scripts Inc. (ACS)也参与其中,Teva将几乎所有面临美国医保Medicare共同支付药物费用的Copaxone患者介绍给该药房。然后Teva再利用ACS、TAF和CDF的信息来计算支付给每个基金会多少钱,以维持每个基金会登记的Copaxone患者的医疗保险共付额的覆盖范围。
ACS与Teva协调将新处方的Copaxone患者转诊到TAF和CDF,在Teva向基金会付款的同时分批转诊患者,这确保Copaxone患者获得TAF和CDF用Teva的钱提供的绝大部分共付额援助。
考虑到美国医保常常是按照比例支付的,在价格提升的过程中,虽然一部分参与整个过程的患者可能支付的价格是相同的,但是医保要支付的钱却逐年上升,这一过程中可以说是薅了医保的羊毛。
而同时对于没有参与这一骗保链条的患者,则可能需要面临被提升了多倍的药价。
后续的药品操纵定价指控则表明,Teva早在2015年12月至2023年5月左右就参与了三次定价阴谋,该公司与其他药品制造商勾结,操纵仿制药价格,操纵投标和分配客户。结果,消费者可能被多收了至少3.5亿美元。
2023年8月,Teva达成了2.25亿美元的延期起诉协议,协议允许该公司避免被强制排除在联邦医疗保健计划之外——潜在的惩罚是Teva选择接受审判并被判有罪。根据协议,该公司将在2024年至2027年期间支付2250万美元,最终在2028年一次性支付1.35亿美元。
参考来源:
https://www.justice.gov/opa/pr/drug-maker-teva-pharmaceuticals-agrees-pay-450m-false-claims-act-settlement-resolve-kickback
https://www.biospace.com/policy/teva-to-pay-450m-in-settlement-with-doj-over-kickback-and-price-fixing-allegations
专利到期
2024-07-01
WASHINGTON, July 1, 2024 /PRNewswire/ -- Vanda Pharmaceuticals Inc. (Vanda) (Nasdaq: VNDA) today announced that the U.S. District Court for the District of Delaware (the "Court") denied the motions for judgment on the pleadings that were filed by Teva Pharmaceuticals USA, Inc. ("Teva") and Apotex Inc. and Apotex Corp. ("Apotex") and ordered that Vanda's HETLIOZ® patent lawsuit may proceed.
Vanda brought this suit in December 2022, alleging patent infringement against Teva and Apotex (the "Defendants"). The Defendants moved for judgment on the pleadings in April 2023. On June 27, 2024, the Court denied the motions. The Court concluded that the issues of patentability were not the same as those resolved in prior patent-infringement litigation between the parties. It further concluded that the Defendants raised issues that require claim construction and fact development before the Court can resolve the case.
Vanda intends to proceed with discovery in the case to continue pursuing its claims. Vanda will request relief from the Court that includes an order requiring the Defendants to discontinue marketing their generic versions of HETLIOZ® until the expiration of the patent-in-suit and enjoining them from the commercial manufacture, use, import, offer for sale and/or sale of these products.
About Vanda Pharmaceuticals Inc.
Vanda is a leading global biopharmaceutical company focused on the development and commercialization of innovative therapies to address high unmet medical needs and improve the lives of patients. For more on Vanda Pharmaceuticals Inc., please visit and follow us on X @vandapharma.
About HETLIOZ®
For full U.S. Prescribing Information for HETLIOZ®, including indication and Important Safety Information, visit .
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Various statements in this press release, including, but not limited to statements regarding Vanda's intentions to proceed with discovery in pursuit of its claims and to request relief from the Court, are "forward-looking statements" under the securities laws. All statements other than statements of historical fact are statements that could be deemed forward-looking statements. Forward-looking statements are based upon current expectations and assumptions that involve risks, changes in circumstances and uncertainties. Important factors that could cause actual results to differ materially from those reflected in Vanda's forward-looking statements include, among others, the results of Vanda's discovery and the Court's willingness to grant Vanda the relief that it seeks. Therefore, no assurance can be given that the results or developments anticipated by Vanda will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on, Vanda. Forward-looking statements in this press release should be evaluated together with the various risks and uncertainties that affect Vanda's business and market, particularly those identified in the "Cautionary Note Regarding Forward-Looking Statements", "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" sections of Vanda's most recent Annual Report on Form 10-K, as updated by Vanda's subsequent Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and other filings with the U.S. Securities and Exchange Commission, which are available at .
All written and verbal forward-looking statements attributable to Vanda or any person acting on its behalf are expressly qualified in their entirety by the cautionary statements contained or referred to herein. Vanda cautions investors not to rely too heavily on the forward-looking statements Vanda makes or that are made on its behalf. The information in this press release is provided only as of the date of this press release, and Vanda undertakes no obligation, and specifically declines any obligation, to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
Corporate Contact:
Kevin Moran
Senior Vice President, Chief Financial Officer and Treasurer
Vanda Pharmaceuticals Inc.
202-734-3400
[email protected]
Jim Golden / Jack Kelleher / Dan Moore
Collected Strategies
[email protected]
SOURCE Vanda Pharmaceuticals Inc.
专利侵权
100 项与 Teva Pharmaceuticals USA, Inc. 相关的药物交易
登录后查看更多信息
100 项与 Teva Pharmaceuticals USA, Inc. 相关的转化医学
登录后查看更多信息
组织架构
使用我们的机构树数据加速您的研究。
登录
或
管线布局
2024年11月20日管线快照
管线布局中药物为当前组织机构及其子机构作为药物机构进行统计,早期临床1期并入临床1期,临床1/2期并入临床2期,临床2/3期并入临床3期
临床前
1
5
临床3期
批准上市
8
34
其他
登录后查看更多信息
药物交易
使用我们的药物交易数据加速您的研究。
登录
或

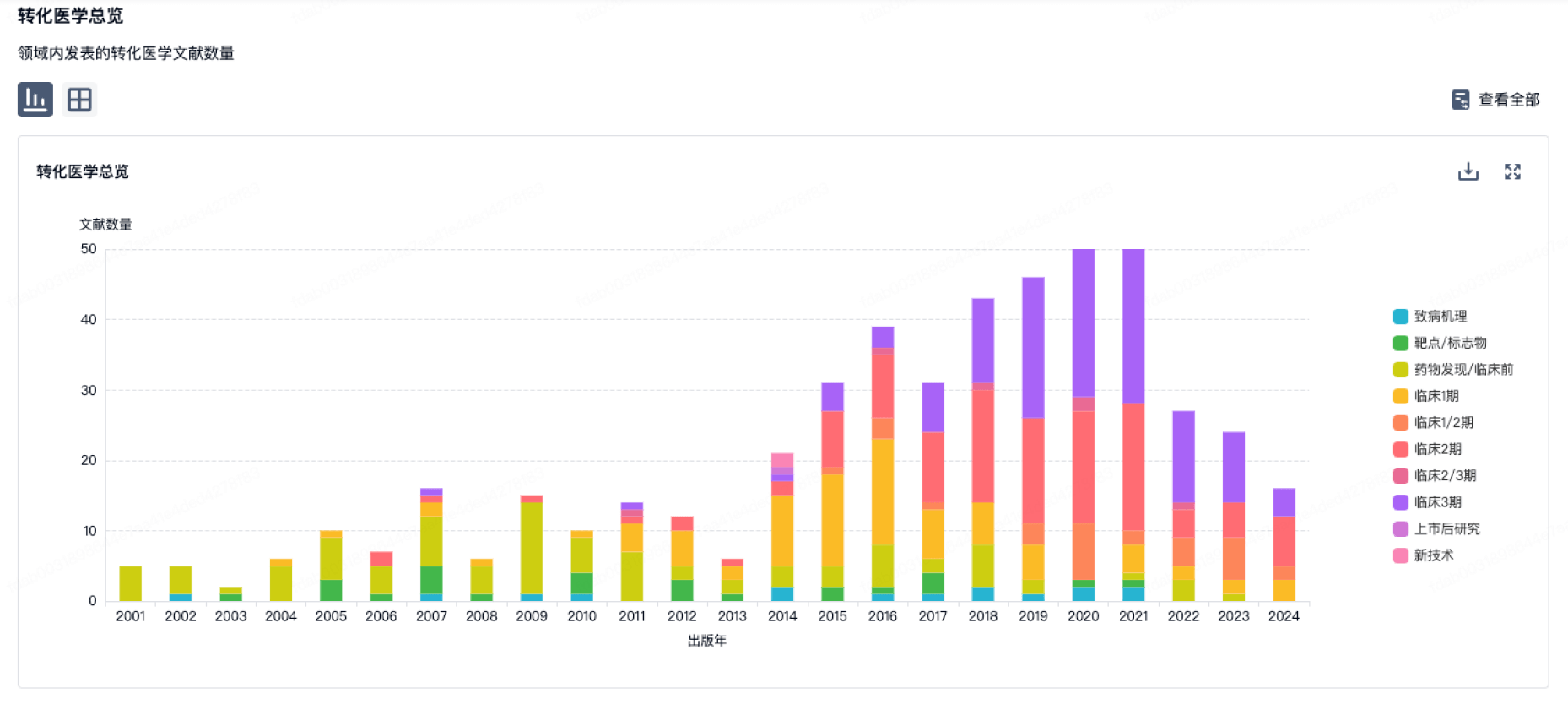
转化医学
使用我们的转化医学数据加速您的研究。
登录
或
营收
使用 Synapse 探索超过 36 万个组织的财务状况。
登录
或
科研基金(NIH)
访问超过 200 万项资助和基金信息,以提升您的研究之旅。
登录
或

投资
深入了解从初创企业到成熟企业的最新公司投资动态。
登录
或

融资
发掘融资趋势以验证和推进您的投资机会。
登录
或

标准版
¥16800
元/账号/年
新药情报库 | 省钱又好用!
立即使用
来和芽仔聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用



