|
|
|
|
|
|
最高研发阶段临床3期 |
首次获批国家/地区- |
首次获批日期- |
靶点- |
作用机制- |
在研机构- |
|
在研适应症- |
|
最高研发阶段无进展 |
首次获批国家/地区- |
首次获批日期- |
A Phase 3, Multicenter, Randomized, Double-Masked, Vehicle-Controlled Clinical Study to Assess the Efficacy and Safety of 5% Tavilermide Ophthalmic Solution for the Treatment of Dry Eye
The purpose of the study is to compare the efficacy and safety of 5% tavilermide ophthalmic solution to placebo for the treatment of the signs and symptoms of dry eye disease.
Multi-Center, Randomized,Double-Masked, Vehicle-Controlled Study to Assess the Safety and Efficacy Study of Tavilermide Ophthalmic Solutions for the Treatment of Dry Eye
The purpose of this study is to assess the safety and efficacy of 5% tavilermide and 1% tavilermide ophthalmic solutions compared with placebo ophthalmic solution in treating the signs and symptoms of dry eye.
A Safety and Efficacy Study of Tavilermide Ophthalmic Solution for the Treatment of Dry Eye Disease
The purpose of this study is to assess the safety and efficacy of Tavilermide Ophthalmic Solution compared with Placebo Ophthalmic Solution in treating the signs and symptoms of dry eye disease.
100 项与 Mimetogen Pharmaceuticals USA, Inc. 相关的临床结果
0 项与 Mimetogen Pharmaceuticals USA, Inc. 相关的专利(医药)
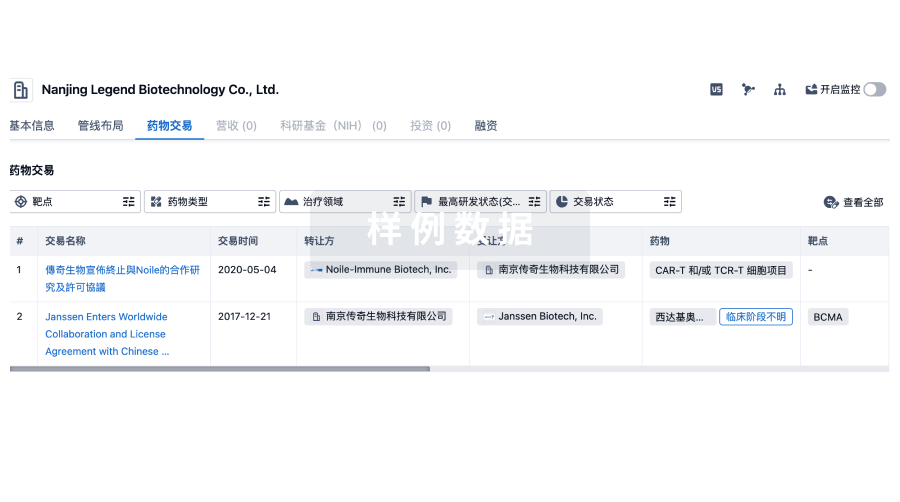
100 项与 Mimetogen Pharmaceuticals USA, Inc. 相关的药物交易
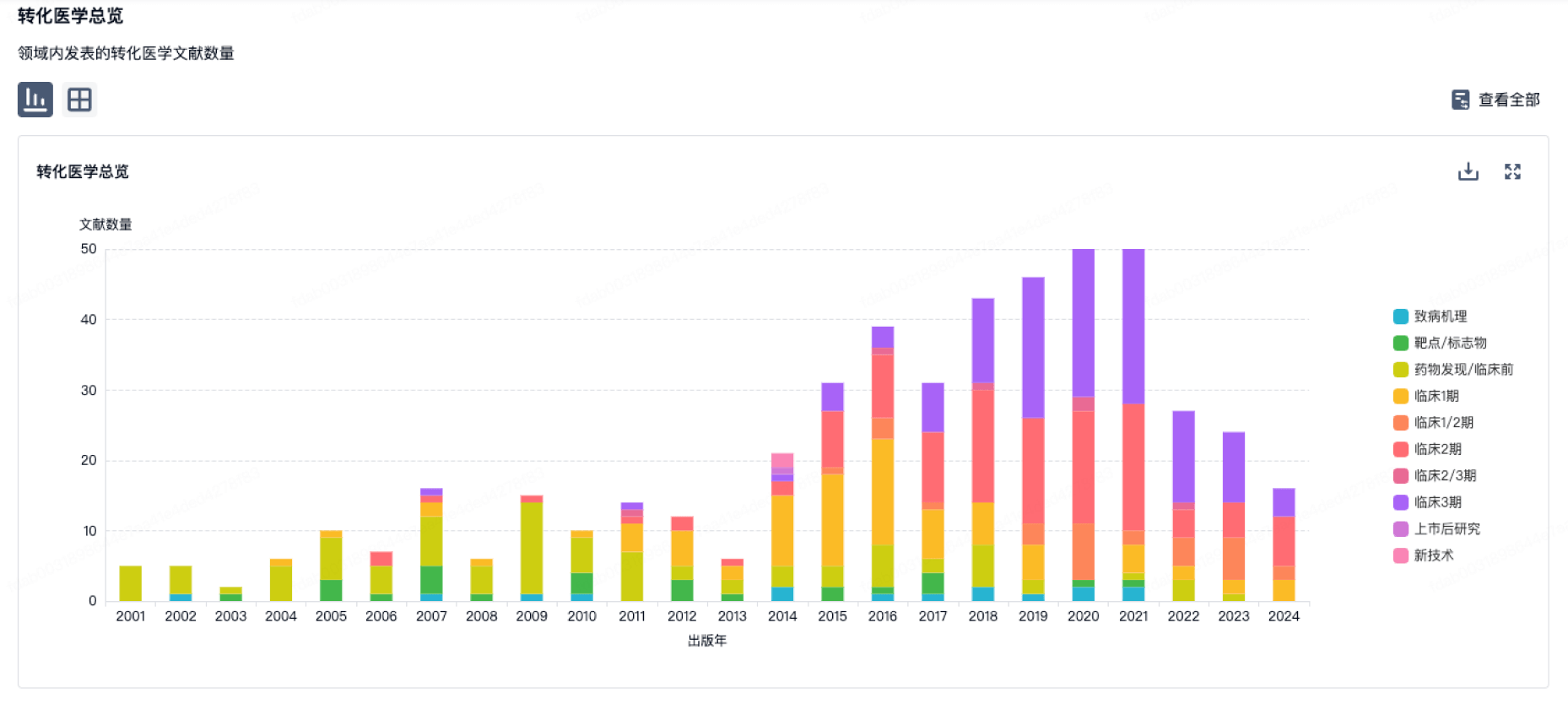
100 项与 Mimetogen Pharmaceuticals USA, Inc. 相关的转化医学