Ultragenyx-Mereo's brittle bone drug sustains fracture rate cut in midphase follow-up
2024-06-12
临床3期临床结果
Preview
来源: FierceBiotech
A new readout offers encouragement as Ultragenyx and Mereo BioPharma advance toward data from the phase 3 portion of the trial.
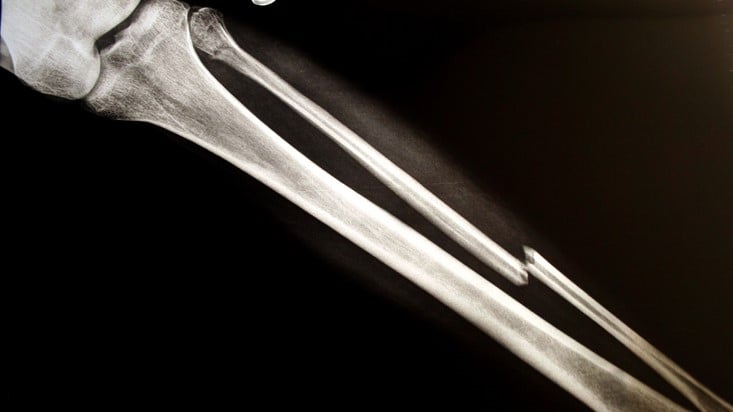
Ultragenyx and Mereo BioPharma have broken off another piece of brittle bone data, linking their drug candidate to sustained reductions in fracture rates in a midphase clinical trial.
The results come from 24 patients with osteogenesis imperfecta who received the anti-sclerostin antibody setrusumab in the first part of the phase 2/3 Orbit study. Over the two years before the trial, the patients had a median annualized fracture rate of 0.72. In October, the partners reported the median annualized fracture rate fell to zero after the participants had received setrusumab for an average of nine months.
Ultragenyx and Mereo’s latest cut of the data looks at outcomes after a mean treatment duration of 16 months. The headline result is a carbon copy of the earlier readout, with the partners again reporting a 67% reduction that kept the median annualized fracture rate down at zero. Pamidronate and zoledronic acid, currently prescribed brittle bone treatments, are associated with lower rates of fracture reduction.
The result is in line with the bar set by Mereo CEO Denise Scots-Knight, who told attendees at a Jefferies event last week that she expected the readout to be “at least comparable, if not better than” the earlier data drop. Shares in Mereo climbed more than 6% to $3.75 in premarket trading, and Ultragenyx’s stock traded up 4% to top $43.
Ultragenyx and Mereo also shared updated bone mineral density (BMD) data. Scots-Knight flagged BMD as a result to watch at the Jefferies event, explaining that her team would be “looking at whether it's increasing in a linear manner or starting to plateau” to inform potential maintenance dosing regimens.
Lumbar spine BMD had increased 22% from baseline after 12 months of treatment without evidence of plateau. The result shows density continued to increase after the six-month analysis, when the partners reported a 14% rise, and suggests further gains are possible as Ultragenyx and Mereo continue to track the patients.
The readout offers encouragement as the partners advance toward data from the phase 3 portion of the trial. Ultragenyx wrapped up enrollment in the phase 3 part of the Orbit study, which recruited people aged 5 to 25 years, and in another phase 3 study in younger children in April. Speaking at a Goldman Sachs event Tuesday, Ultragenyx CEO Emil Kakkis said an interim analysis is due around the end of 2024.
更多内容,请访问原始网站
文中所述内容并不反映新药情报库及其所属公司任何意见及观点,如有版权侵扰或错误之处,请及时联系我们,我们会在24小时内配合处理。
靶点
-药物
热门报告
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。