FDA approves first-ever therapy for Demodex blepharitis
2023-07-26
上市批准临床2期临床3期临床结果

Preview
来源: Pharmaceutical Technology
Adam Zamecnik
@a_zamecnik
Preview
来源: Pharmaceutical Technology
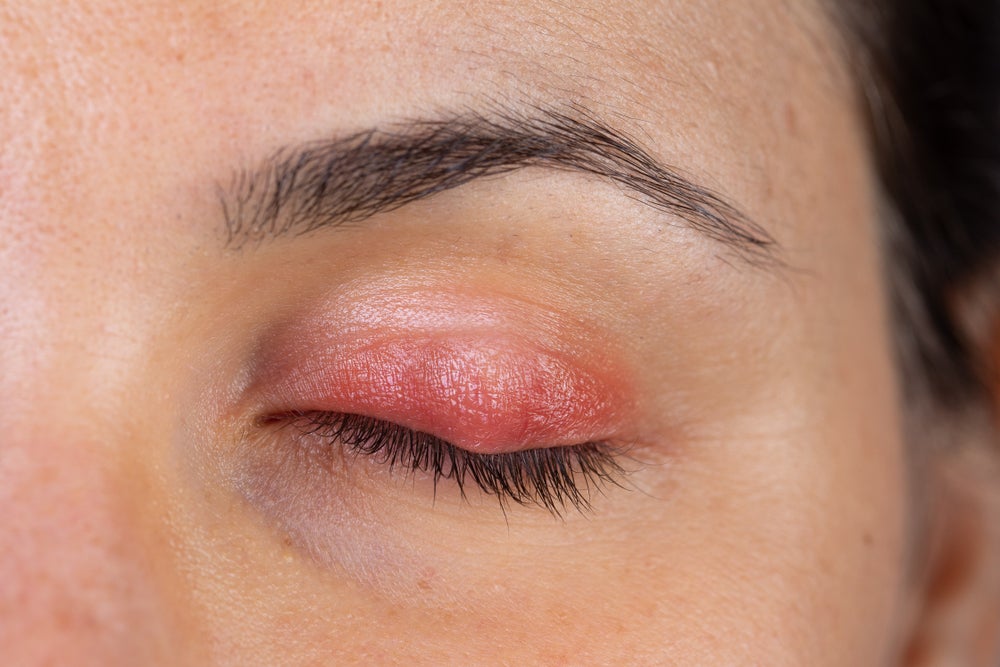
Blepharitis is an eye condition that results in eyelid inflammation, redness, and irritation. Credit: Shutterstock / sruilk.
The US Food and Drug Administration (FDA) has approved Tarsus Pharmaceuticals’ Xdemvy for the treatment of Demodex blepharitis, marking the first-ever FDA approval of a drug for the condition that affects the eyelids.
The treatment is expected to be available for prescription by the end of August, per the announcement. Prior to this approval, tea tree oil was used as a treatment for the condition. However, the use of tea tree oil came with its own safety issues at higher concentrations.
Recommended Reports

Preview
来源: Pharmaceutical Technology
ReportsLOA and PTSR Model - Momelotinib Dihydrochloride in Post-Polycythemia Vera Myelofibrosis (PPV-MF) GlobalData

Preview
来源: Pharmaceutical Technology
ReportsLOA and PTSR Model - Quizartinib Dihydrochloride in Myelodysplastic Syndrome GlobalData
View all
Xdemvy is expected to cost $1,850 per year, with a patient out-of-pocket cost estimated to be $100 or less, based on the company’s July presentation. The company projects the drug to have more than $1bn in peak net sales potential. According to consensus forecasts from GlobalData’s Pharma Intelligence Centre, Xdemvy is estimated to earn $652m in global sales in 2029. GlobalData is the parent company of Pharmaceutical Technology.
Blepharitis is an eye condition that results in eyelid inflammation, redness, and irritation. While blepharitis can be caused by other factors, Demodex blepharitis is specifically caused by Demodex mites, a type of microscopic parasite that infest human hair follicles. Demodex blepharitis affects roughly 25 million eye care patients in the US, which translates into one out of every 12 adults, based on the 25 June announcement.
The agency’s decision is based on data from the Phase IIb/Phase III Saturn-1 (NCT04475432) and Phase III Saturn-2 (NCT04784091) studies. Both trials have met their primary endpoints, reporting significant reductions in the number of upper lid collarettes, per the 25 June announcement. Additionally, the trials reported statistically significant improvements in mite eradication.
The company also plans to develop a preservative-free formulation of Xdemvy against the same condition. The drug is also being developed for use in meibomian gland disease. Tarsus expects to share topline data from a Phase IIa trial of Xdemvy in meibomian gland disease in H2 2023, per the company’s June corporate presentation. Tarsus is working with the biotech LianBio to develop Xdemvy in Demodex blepharitis and meibomian gland disease in China.
更多内容,请访问原始网站
文中所述内容并不反映新药情报库及其所属公司任何意见及观点,如有版权侵扰或错误之处,请及时联系我们,我们会在24小时内配合处理。
靶点
-热门报告
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。