预约演示
更新于:2025-05-07
Bronchitis, Chronic
慢性支气管炎
更新于:2025-05-07
基本信息
别名 BRONCHITIS CHRONIC、BRONCHITIS, CHRONIC、Bronchitis chronic + [28] |
简介 A subcategory of CHRONIC OBSTRUCTIVE PULMONARY DISEASE. The disease is characterized by hypersecretion of mucus accompanied by a chronic (more than 3 months in 2 consecutive years) productive cough. Infectious agents are a major cause of chronic bronchitis. |
关联
131
项与 慢性支气管炎 相关的药物靶点 |
作用机制 IL-4Rα抑制剂 |
在研机构 |
最高研发阶段批准上市 |
首次获批国家/地区 中国 |
首次获批日期2024-09-10 |
靶点- |
作用机制- |
在研机构 |
原研机构 |
非在研适应症- |
最高研发阶段批准上市 |
首次获批国家/地区 中国 |
首次获批日期2020-08-28 |
靶点- |
作用机制- |
在研机构 |
原研机构 |
非在研适应症- |
最高研发阶段批准上市 |
首次获批国家/地区 中国 |
首次获批日期2020-05-31 |
644
项与 慢性支气管炎 相关的临床试验ChiCTR2500100047
Prospective, multicenter, single blind, randomized controlled clinical trial evaluating the efficacy and safety of pulsed electric field ablation system for the treatment of patients with chronic obstructive pulmonary disease characterized by chronic bronchitis
开始日期2025-04-07 |
申办/合作机构 |
NCT06891274
Study on the Lung Aerosol Deposition of Ultrasonic Rock Salt Inhalation Therapy
In the in vitro modeling phase, the investigators plan to use a realistic human upper respiratory tract model and an asymmetric ideal bronchial tree model. By combining 3D printing experiments with CFD simulations, the investigators will investigate the respiratory tract deposition rate and distribution of micro - moist particles in ultrasound rock salt aerosol inhalation therapy. This will be compared with a small - volume nebulizer to verify the effectiveness of ultrasound rock salt aerosol inhalation therapy and lay the foundation for further research on the application of this inhalation technique to other drug formulations.
In the clinical phase, the investigators plan to have subjects inhale radioactive aerosols and use PET/CT imaging technology to assess the deposition rate of aerosol particles in the lungs. By quantitatively analyzing the images, the investigators will study their distribution characteristics in real human bodies to verify the accuracy of the in vitro models and the potential for clinical application.
In the clinical phase, the investigators plan to have subjects inhale radioactive aerosols and use PET/CT imaging technology to assess the deposition rate of aerosol particles in the lungs. By quantitatively analyzing the images, the investigators will study their distribution characteristics in real human bodies to verify the accuracy of the in vitro models and the potential for clinical application.
开始日期2025-03-31 |
申办/合作机构 |
CTRI/2025/03/082278
Clinical evaluation of ultrasound guided bilateral tap block versus intraperitoneal instillation of levobupivacaine for post operative analgesia in laparoscopic abdominal surgeries. - nil
开始日期2025-03-23 |
100 项与 慢性支气管炎 相关的临床结果
登录后查看更多信息
100 项与 慢性支气管炎 相关的转化医学
登录后查看更多信息
0 项与 慢性支气管炎 相关的专利(医药)
登录后查看更多信息
11,208
项与 慢性支气管炎 相关的文献(医药)2025-12-31·Pulmonology
Issue 3—The occupational burden of respiratory diseases, an update
Review
作者: Muñoz, X. ; Costa, J.T. ; Moitra, S. ; Murgia, N. ; Akgun, M. ; Ferreira, A.J. ; Blanc, P.D. ; Toren, K.
2025-07-01·Journal of Biotechnology
Fungal elicitors increase cell biomass, pyrroloquinazoline alkaloids production and gene expression levels of biosynthetic pathways in Adhatoda vasica Nees cell cultures
Article
作者: Singh, Bharat ; Sharma, Ram Avtar ; Saxena, Anuja
2025-04-01·Respiratory Medicine
Bacterial colonisation doubles the risk of exacerbation in alpha-1 antitrypsin deficiency
Article
作者: Stockley, Robert A ; Spittle, Daniella A ; Turner, Alice M ; Stanka, Jan ; De Soyza, Joshua ; Pye, Anita
306
项与 慢性支气管炎 相关的新闻(医药)2025-05-05
·汇聚南药
作者:Gcplive来源:药评中心宋伯今年65岁,一直在吃硝苯地平缓释片,将血压稳定在140/90mmHg。这几天,他的血压连续升高至180/110 mmHg,头晕脑胀,连忙去社区医院就诊。在医院,医生询问宋伯有没有睡眠、情绪等变化,都被一一否定了。进一步询问有没有吃其他药,宋伯才想起来,半个月前自己莫名咳嗽,他跑了好几家药店,都说没卖复方甘草片,最后才在城中村的小药房买到。买到药后,他一天3次,一次4片,连续吃了一星期。医生表示,问题就出在复方甘草片上。对高血压患者来说,长期超量服用复方甘草片,会引起“假性醛固酮增多症”,使血压突然升高。此外,甘草片很可能引起其他问题,自己买来吃很不安全。一、甘草片曾是家庭常备药曾经复方甘草片的身影到处都是,尤其是季节交替的时候,几乎每个家庭都备有一小瓶,咳嗽了就吃几天,起效很快。复方甘草片的性价比高,止咳效果好,被老百姓称为“平价神药”。甘草其实不是一种草,而是甘草、胀果甘草、光果甘草等植物的根茎,经过晾晒、炮制而成。很早以前,甘草就被当做药物的配料。《神农百草经》记载甘草:“主五脏六腑寒热邪气,坚筋骨,长肌肉,倍力,金创,解毒。久服轻身延年。”《本草纲目》也提到:“诸药中甘草为君”,指出甘草不仅可以止咳、镇痛、解毒,还可以中和其他药物的毒性。从古至今,甘草在民间都有很广的流传度。二、为什么复方甘草片买不到了?经常用药的人可能会发现,在以前,随便一家药店都能买到复方甘草片,后来复方甘草片逐渐消失在市面上,几乎没有药店售卖,这是为什么呢?复方甘草片为含有特殊药品的复方制剂,每片含甘草浸膏粉 112.5 毫克、阿片粉4毫克、樟脑 2 毫克、八角茴香油 2 毫克、苯甲酸钠 2 毫克。其实早在2005年,复方甘草片就药监局被归为处方药了,不能自行购买,需在医生的指导下使用。2014年,媒体报道有华人携带复方甘草片赴美被扣下,最终被判5年内不得入境,原因是复方甘草片中含有未经FDA批准使用的违禁成分,引起轩然大波。2020年,为进一步保障用药安全,国家药监局发布了《关于修订复方甘草片说明书的公告》,对复方甘草片说明书中的“不良反应”和“注意事项”等内容进行了修订。复方甘草片最大的争议,源于它的成瘾性。临床研究和病例显示,长期服用复方甘草片可能会产生药物依赖性和成瘾性,一旦停药,就会出现流鼻涕、冒冷汗、烦躁不安的戒断症状,需要2-3个月才能恢复正常。因此,医生一般建议连续服用不超过1周。复方甘草片成瘾性的“罪魁祸首”是阿片粉,有较强的镇咳作用,但老百姓知之甚少。此外,长期超量服用复方甘草片,还会引发假性醛固酮增多症,一种继发性高血压,表现为血压升高、血钾偏低、代谢性碱中毒等,如不及时治疗,可导致肾衰、心衰,甚至周期性瘫痪。三、4类人切勿随意服用即使复方甘草片被列为处方药,不能随意购买,但依然有很多人痴迷于它的镇咳效果,想尽办法自行服用。厦门大学附属中山医院药学部主任欧阳华接受采访时表示,药物依赖的严重性不亚于毒品,贪便宜的损失也许更大。如果以下4类患者擅自服用,可能产生严重的后果。1、婴幼儿、老年人阿片成分在婴幼儿和老年人的体内代谢比较慢,半衰期长,血药浓度过高可能引起呼吸困难,危及生命。婴幼儿、老年人如需服用复方甘草片,一定要有医生的指导,按照医嘱用药。2、高血压患者复方甘草片中含有甘草酸。甘草酸可水解为甘草次酸,甘草次酸可抑制氢化可的松氧化酶(11-β-羟甾醇脱氢酶),造成体内氢化可的松水平升高,从而引起水钠潴留和低血钾。高血压患者服用复方甘草片之后,会出现血压升高、血钾偏低等症状,不利于血压稳定。3、消化性溃疡患者慢性支气管炎、十二指肠溃疡等消化性溃疡的患者不宜使用,复方甘草片具有糖皮质激素样作用,可以使胃酸的分泌量增加、胃粘液的分泌量减少,加重病情。4、糖尿病患者同样的,复方甘草片具有糖皮质激素样作用,与抗糖药合用时,会发生拮抗作用,影响抗糖药的药效,不利于血糖的稳定,甚至导致病情加重。再次提醒:复方甘草片中的阿片会成瘾,甘草中的甘草次酸具有糖皮质激素样作用,长期服用会升高血压! 喜欢我们文章的朋友点个“在看”和“赞”吧,不然微信推送规则改变,有可能每天都会错过我们哦~免责声明“汇聚南药”公众号所转载文章来源于其他公众号平台,主要目的在于分享行业相关知识,传递当前最新资讯。图片、文章版权均属于原作者所有,如有侵权,请在留言栏及时告知,我们会在24小时内删除相关信息。信息来源:药评中心往期推荐本平台不对转载文章的观点负责,文章所包含内容的准确性、可靠性或完整性提供任何明示暗示的保证。
2025-05-03
unsetunset一、 研究摘要unsetunset背景:如何利用大规模通用语言模型(LLM)来辅助疾病诊断,以提高诊断的准确性和效率挑战: LLM在临床诊断中的有效性尚未得到充分验证; 如何确保LLM的输出与临床实践标准一致。解决方案:提出MedFound,一个具有1760 亿参数的通用医学语言模型使用的技术:预训练,微调,对齐结果:在分布内、分布外和长尾分布方面优于其他基线LLMsunsetunset二、研究背景unsetunset1. 医疗诊断的重要性与挑战准确诊断是医疗核心,但初级保健误诊率高达20%,导致17%的医疗不良事件。传统临床决策支持系统(CDSS)和机器学习依赖结构化数据,开发复杂且临床落地困难。2. 语言模型在医疗领域的潜力与局限PLMs及LLMs在自然语言处理中表现突出,催生了ClinicalBERT、BioGPT等生物医学专用模型。现有研究集中于用例报告(如ChatGPT),缺乏针对真实临床场景设计的LLM存在生成幻觉风险,需通过对齐技术确保LLM的安全性与准确性。unsetunset三、研究材料与方法unsetunset1. 数据集研究主要使用了三大知识图谱:MSI、HetioNet和KEGG,每个图谱中包含的药物、疾病、基因和通路等数量如下表所示:表1.数据集来源及用途2. 模型框架3. 微调过程这是模型训练的流程图。第一阶段,基于 PMC - CR、Med - Text、MedDX - Note、MIMIC - 3 - Note 和真实世界数据,采用低秩适应(LORA)对BLOOM - 176B进行微调,这种微调方式能显著减少可训练参数。第二阶段,采用自举式数据增强对 MedFound 进行微调,目的是让模型模仿医生的诊断推理过程,进而产生 MedFound - DX。此阶段主要使用 MedDX - FT 数据集,该数据集包含医疗记录和相关诊断原理演示。使用少量种子数据集(有医生诊断的推断过程),采用一种自引导策略,在无需大量专家劳动的情况下,为每个电子健康记录自动生成高质量诊断理由(中间推理步骤)。第三阶段是偏好对齐,该框架集成了 “诊断层次结构偏好” 和 “实用性偏好”。目的是将LLM生成内容与标准临床实践对齐。步骤 1.1:初始微调(Seed Dataset Fine-tuning)输入:基础语言模型(Language Model, M) + 种子数据集(D₀)109,364条记录操作:使用医生标注的小规模种子数据集 D₀(包含病例输入和专家诊断理由)对模型 M 进行微调,得到初步优化模型 M'目标:让模型学会模仿医生的诊断推理逻辑(如症状分析、鉴别诊断等)。步骤 1.2:自举生成增强数据(Bootstrapping Augmented Data)输入:微调后的模型 M' + 无标注原始数据(D_raw)。操作:对每个病例输入,模型 M' 生成两种诊断理由:自由生成(r₁):仅基于病例文本直接生成诊断理由。提示生成(r₂):在已知正确诊断(作为提示)的条件下生成理由。选择策略:通过评分函数 s(r) 比较 r₁ 与 r₂,保留质量更高的理由,构建增强数据集 D₁。意义:利用模型自身生成高质量数据,解决标注数据不足的问题。步骤 1.3:二次微调(Augmented Data Fine-tuning)输入:模型 M' + 增强数据集 D₁。操作:使用 D₁ 对 M' 进一步微调,得到最终微调模型 M''目标:强化模型对复杂病例的诊断推理能力,减少错误生成。4. 偏好对齐偏好对齐过程包括两个步骤:偏好构建和偏好优化。4.1 诊断层级偏好构建借助ICD的指导,以解决仅基于诊断正确性设置偏好时可能出现的问题,因为这种方式可能会导致信号稀疏,尤其是在涉及罕见疾病或难以诊断的病症时。例如,ICD编码E11(2型糖尿病)是几个子编码的父编码,包括E11.0(伴有高渗状态的2型糖尿病)、E11.1(伴有酮症酸中毒的2型糖尿病)和E11.2(伴有肾脏并发症的2型糖尿病)。这些子ICD编码在语义上彼此之间的差异比它们与父编码E11的差异更大。ICD的层级结构有助于基于模型输出与ICD编码的对齐,构建更细致的偏好。从响应中提取诊断标签,并将其映射到ICD编码,与真实的ICD编码标签进行比较,从而定义精确程度。如果和在ICD层级结构中有更细粒度的共同父节点,那么的值就越高。给定带有标签的输入,以及两个生成输出和,其映射的ICD编码分别为和,如果,则在训练中使用偏好,表示比更受偏好。4.2 有用性偏好构建作者构建一个评分模型,该模型在包含标注为“有用”或“无用”的诊断依据的专家注释数据集上进行训练。训练一个二元分类模型作为评分模型,以评估每个诊断依据的有用程度。对于给定的输入和响应,使用以下损失函数训练二元分类器:其中代表专家注释的标签,代表预测概率。概率被用作有用性得分。给定输入和两个生成输出和,如果,则在训练中使用偏好,其中是概率得分。4.3 偏好优化通过直接偏好优化(DPO)实现多个偏好目标的优化。 诊断层级偏好和有用性偏好被联合训练。给定一份医疗记录,对多个响应进行采样。我们通过两种方式选择偏好对:一种是随机选择两个响应,另一种是选择具有最高精确程度(基于ICD编码)的两个响应,从而得到两个用于偏好优化的偏好对。 对于一对响应,如果它们的精确程度不同,则使用诊断层级偏好;否则,使用有用性偏好。目标函数为:其中是输入提示,和分别表示更受偏好和不受偏好的响应,是参考策略,是带有参数的最优策略,是一个控制与参考策略偏差程度的参数。5. 模型评估设计为了评估模型与其他基线模型在实际场景中的诊断性能作者在分布内(ID)、分布外(OOD) 和长尾疾病分布环境中进行了评估,涵盖八个专业的疾病,包括肺病学、胃肠病学、泌尿学、心脏病学、免疫学、精神病学、神经病学和内分泌学(图4左)。同时作者还对AI 系统进行了临床应用的评估,首先对比了人类医生、AI、AI辅助人类医生的诊断准确率,随后在人类评估框架下对AI在理解力、推理能力、决策支持等多方面进行了定性研究(图4右)unsetunset四、研究结果与讨论unsetunset1. 模型分布内及分布外的性能对比作者首先评估了MedFound-DX-PA在ID(分布内)和OOD(分布外)设置中诊断常见疾病的性能,并将其与几种领先的大型语言模型(LLMs)进行了比较,包括开源的MEDITRON-70B、Clinical Camel-70B和Llama 3-70B,以及闭源的GPT-4o。MEDITRON-70B和Clinical Camel-70B是经过医学预训练的LLMs,在医疗任务中表现出色;Llama 3-70B是流行的开源Llama系列的一员,在各种领域特定任务中表现优异;而GPT-4o作为ChatGPT的最新版本,具有更广泛的知识库和增强的问题解决能力,在诊断任务中显示出潜力。在ID设置下,研究者构建了MedDX-Test数据集,评估了MedFound-DX-PA在诊断常见细粒度疾病方面的性能,结果显示其Top-3平均准确率达到84.2%(图5a),显著优于其他四种模型,并且在所有专科中的准确率均表现优异,范围在82.4%到89.6%之间(图5b)。在OOD设置下,研究者使用MedDX-OOD数据集进一步评估了模型的泛化能力,该数据集包含从外部真实环境中收集的病例,作者的模型作为诊断通用模型在多种临床疾病中具有泛化能力,尤其是在细粒度疾病诊断方面。(图5c-d)。结果表明,MedFound-DX-PA在所有专科中均显著优于基线模型,充分证明了其在多种临床疾病诊断中的广泛泛化能力,特别是在细粒度疾病诊断方面的卓越表现。作者通过提示其进行特定于专业的设置,将疾病专家的角色分配给各个大模型,在MedDX-Test和MedDX-OOD数据集上的Top-3准确率分别达到87.9%-93.9%和85.8%-90.2%(图6),表明其能够适应专科设置的精度要求。与现有的专科决策支持工具相比,MedFound的性能相当或更优,进一步证明了其作为诊断通用模型在专科领域的潜力。2. LLMS在罕见病方面的表现作者进一步评估LLMs在诊断长尾分布罕见疾病中的性能。作者指出疾病分布呈长尾分布,常见疾病覆盖 99% 的人口,其余 1% 包含多种不太常见的疾病(图6a)。作者评估了上述八大类疾病中的2,105 种罕见疾病(图6b),MedFound-DX-PA性能也均超过了其他基线模型(图6c,其中条形图表示 MedFound-DX-PA 对每个专业中个别疾病的前 3 名准确性。)在8类罕见病的诊断上,MedFound-DX-PATop-3准确率从也均超过了其他基线模型(图7d)。3. LLM 与医生之间的性能比较作者将基于 LLM 的诊断系统与内分泌学和肺病学人类医生的诊断能力进行了比较。招募了 18 名医生,包括 9 名内分泌科医生和 9 名肺科医生,并按临床经验进一步分为三组:初级 (n = 3) 、中级 (n = 3) 和高级 (n = 3)。每位医生被分配了 150 例进行诊断。根据专家小组建立的金标准诊断来衡量性能。在肺病学方面,MedFound-DX-PA 的诊断准确率为 72.6%,超过初级医生 (60.0%) 和中级医生 (67.7%),但略低于高级医生 (76.2%),在内分泌学中,AI 的准确性 (74.7%) 超过了初级医生 (69.4%) 和中级医生 (72.5%),与高级医生 (75.2%) 相似。当提供 EHR 记录(删除诊断)时,来自两个专业的初级和中级医生进行了初步诊断。两周后,他们参考 AI 生成的内容进行第二个诊断(图8深蓝色柱子)肺病学方面,人工智能辅助大大提高了初级和中级医生的准确性,分别提高了 11.9% 和 4.4%,性能接近人工智能系统,但仍略低于高级医生。在内分泌学方面,在 AI 辅助下,初级和中级内分泌学家组的准确率分别大幅提高到 74.0%(增加 4.6%)和 78.8%(增加 6.3%)。医生最初根据患者目前的病史和实验室检查的 C 反应蛋白水平诊断为“急性支气管炎”。然后,在 AI 生成的内容的帮助下,强调患者的复发性支气管炎病史,医生将诊断修改为“慢性支气管炎急性加重”的准确诊断。当医生在患者的实验室检查中观察到促甲状腺激素水平升高时,就做出了亚临床甲状腺功能减退症的初步诊断。在 AI 辅助下进行重新评估期间,该模型强调了以前被忽视的抗甲状腺过氧化物酶抗体水平升高,表明可能存在潜在的自身免疫性甲状腺疾病。因此,医生将诊断修改为“自身免疫性甲状腺炎”。4. AI 诊断能力的人类评估框架为了解决传统评估指标(如准确率或自然语言生成评分)无法全面捕捉诊断过程临床质量的问题,作者提出了CLEVER评估框架,涵盖八项临床指标,包括医学案例理解、临床推理、医学指南和共识、鉴别诊断相关性、诊断可接受性、不忠实内容、偏见与不公平性、潜在危害性。通过六名资深医生使用Likert评分系统(1-5分)进行评估,MedFound-DX-PA在多项指标上显著优于未对齐的LLM模型。结果表明,通过与人价值观对齐,LLM系统可以优化其可信度和临床适用性,为临床决策提供更可靠的支持。MedFound-DX-PA在多项临床指标上的优异表现,展示了其作为诊断通用模型在真实世界诊断中的潜力。5. 训练组件对 LLM 性能的影响为了探讨MedFound及其关键组件对LLMs(大型语言模型)诊断性能的影响,作者使用MedDX-Bench数据集进行了实验,比较了MedFound与Clinical Camel-70B、Llama-3-70B和MEDITRON-70B的表现。通过MED-Prompt方法评估LLMs在MedDX-Test、MedDX-OOD和MedDX-Rare数据集上的表现。MedFound(未使用SC解码)在微准确率上显著优于其他LLMs,分别高出14.4%、11.9%和11.1%。通过领域特定数据的微调,所有模型在MedDX-Bench任务上的表现均有显著提升。在MedDX-Test、MedDX-OOD和MedDX-Rare上的微准确率分别提高了14.9%、15.9%和12.7%。unsetunset五、不足与可借鉴点unsetunset不足模型规模过大(176B参数),部署和维护成本高缺乏模型压缩和轻量化方案的探讨可借鉴点提出自举策略解决标注数据不足问题思维链(CoT)微调设计统一偏好对齐框架,对齐LLM与人类对诊断的理解参考文献:Wang, G., Liu, X., Liu, H., Yang, G. et al. A Generalist Medical Language Model for Disease Diagnosis Assistance. Nat Med (2025)文章代码:https://github.com/medfound/medfound投稿人:李 坤责任编辑:许燕红
2025-05-01
24 abstracts, including 1 oral presentation and 4 late-breaking posters on Dupixent, to showcase new clinical and real-world analyses in chronic obstructive pulmonary disease (COPD) and asthma
COPD data from the landmark Phase 3 trials will highlight Dupixent impact on lung function and health-related quality of life across broad populations of patients with type 2 inflammation
Asthma abstracts include late-breaking data on mucus burden and the first presentation of efficacy results from a Phase 2 trial designed to study Dupixent in allergic bronchopulmonary aspergillosis (ABPA) in patients with asthma
TARRYTOWN, N.Y. , May 01, 2025 (GLOBE NEWSWIRE) -- Regeneron Pharmaceuticals, Inc. (NASDAQ: REGN) today announced 24 abstracts on Dupixent® (dupilumab) clinical data and real-world analyses in respiratory diseases will be presented at the American Thoracic Society (ATS) International Conference 2025 being held from May 18 to 21 in San Francisco, California . The abstracts, presented in collaboration with Sanofi, demonstrate the benefit of targeting IL-4 and IL-13 to address type 2 inflammation in chronic obstructive pulmonary disease (COPD) and asthma – chronic respiratory diseases that can impair lung function and impact daily life.
“The data presented at ATS demonstrate Regeneron’s commitment to advancing the scientific understanding of type 2 inflammation across chronic respiratory diseases to ultimately transform care and quality of life for as many appropriate patients as possible,” said Jennifer Maloney , M.D., Therapeutic Area Lead of Immune, Inflammation, and Infectious Disease Global Development at Regeneron . “Among our 24 abstracts at ATS are the latest results from the Dupixent COPD program, which include new analyses of its impact on critical disease measures such as lung function in broad patient populations with type 2 inflammation. We also look forward to sharing new asthma insights in both adult and pediatric populations.”
COPD data assess Dupixent impact on lung function and exacerbations in COPD, including patients with or without emphysema Notable abstracts in COPD will highlight new results from the pivotal landmark Phase 3 BOREAS and NOTUS trials, including analyses demonstrating Dupixent reduced exacerbations and improved lung function regardless of whether patients had emphysema. In the pivotal COPD trials, the majority of patients had chronic bronchitis (≥95%) and ≥30% had emphysema. Additional data being presented also demonstrate Dupixent improved multiple spirometry measures of lung function that were sustained through 52 weeks, compared to placebo.
Furthermore, a late-breaking poster of a win-ratio post-hoc analysis will assess the likelihood of avoiding a composite of events including death, hospitalization, worsening symptoms and lung function decline in the COPD pivotal trials by comparing each patient on Dupixent to each patient on placebo.
The safety results from BOREAS and NOTUS COPD trials were generally consistent with the known safety profile of Dupixent in its other approved indications. In pooled data from both trials, the most common adverse events (AEs; ≥2%) more frequently observed with Dupixent than placebo were viral infection, headache, nasopharyngitis, back pain, diarrhea, arthralgia, urinary tract infection, local administration reaction, rhinitis, eosinophilia, toothache and gastritis.
Asthma data reinforce impact of Dupixent on mucus burden, exacerbations and disease control A late-breaking poster on the VESTIGE imaging trial will highlight that Dupixent reduced mucus burden, compared to placebo, as measured by mucus plug scores and volume regardless of fractional exhaled nitric oxide (FeNO) levels. An analysis of the VOYAGE trial also shows that, in children aged 6 to 11 years, Dupixent reduced exacerbations and improved disease control regardless of how long they had the disease.
The safety results in the asthma trials were generally consistent with the known safety profile of Dupixent in moderate-to-severe asthma, with the addition of helminth infections in the VOYAGE trial. In VOYAGE, the most common AEs more frequently observed with Dupixent than placebo were injection site reactions, viral upper respiratory tract infections and eosinophilia. In VESTIGE, the most common AEs (≥5%) more frequently observed with Dupixent than placebo included COVID-19 and injection site reactions. Results will also be shared for the first time in an oral presentation from the Phase 2 AIRED trial evaluating the impact of Dupixent on lung function, exacerbations and health-related quality of life in adults and adolescents with allergic bronchopulmonary aspergillosis (ABPA) and asthma. ABPA is a progressive lung disease caused by hypersensitivity to a fungal microorganism that can live in the airways of patients with breathing disorders like asthma.
The full list of Regeneron and Sanofi presentations at ATS includes:
About DupixentDupixent, which was invented using Regeneron’s proprietary VelocImmune® technology, is a fully human monoclonal antibody that inhibits the signaling of the interleukin-4 (IL-4) and interleukin-13 (IL-13) pathways and is not an immunosuppressant. The Dupixent development program has shown significant clinical benefit and a decrease in type 2 inflammation in Phase 3 trials, establishing that IL-4 and IL-13 are two of the key and central drivers of the type 2 inflammation that plays a major role in multiple related and often co-morbid diseases.
Dupixent has received regulatory approvals in more than 60 countries in one or more indications including certain patients with atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyps (CRSwNP), eosinophilic esophagitis (EoE), prurigo nodularis, chronic spontaneous urticaria (CSU) and chronic obstructive pulmonary disease (COPD) in different age populations. More than 1,000,000 patients are being treated with Dupixent globally.1
About Regeneron’s VelocImmune Technology Regeneron 's VelocImmune technology utilizes a proprietary genetically engineered mouse platform endowed with a genetically humanized immune system to produce optimized fully human antibodies. When Regeneron 's co-Founder, President and Chief Scientific Officer George D. Yancopoulos was a graduate student with his mentor Frederick W. Alt in 1985, they were the first to envision making such a genetically humanized mouse, and Regeneron has spent decades inventing and developing VelocImmune and related VelociSuite® technologies. Dr. Yancopoulos and his team have used VelocImmune technology to create a substantial proportion of all original, FDA-approved fully human monoclonal antibodies. This includes Dupixent® (dupilumab), Libtayo® (cemiplimab-rwlc), Praluent® (alirocumab), Kevzara® (sarilumab), Evkeeza® (evinacumab-dgnb), Inmazeb® (atoltivimab, maftivimab and odesivimab-ebgn) and Veopoz® (pozelimab-bbfg). In addition, REGEN-COV® (casirivimab and imdevimab) had been authorized by the FDA during the COVID-19 pandemic until 2024.
Dupilumab Development Program Dupilumab is being jointly developed by Regeneron and Sanofi under a global collaboration agreement. To date, dupilumab has been studied across more than 60 clinical trials involving more than 10,000 patients with various chronic diseases driven in part by type 2 inflammation.
In addition to the currently approved indications, Regeneron and Sanofi are studying dupilumab in a broad range of diseases driven by type 2 inflammation or other allergic processes in Phase 3 trials, including chronic pruritus of unknown origin, bullous pemphigoid and lichen simplex chronicus. These potential uses of dupilumab are currently under clinical investigation, and the safety and efficacy in these conditions have not been fully evaluated by any regulatory authority.
U.S. INDICATIONS DUPIXENT is a prescription medicine used:
to treat adults and children 6 months of age and older with moderate-to-severe eczema (atopic dermatitis or AD) that is not well controlled with prescription therapies used on the skin (topical), or who cannot use topical therapies. DUPIXENT can be used with or without topical corticosteroids. It is not known if DUPIXENT is safe and effective in children with atopic dermatitis under 6 months of age. with other asthma medicines for the maintenance treatment of moderate-to-severe eosinophilic or oral steroid dependent asthma in adults and children 6 years of age and older whose asthma is not controlled with their current asthma medicines. DUPIXENT helps prevent severe asthma attacks (exacerbations) and can improve your breathing. DUPIXENT may also help reduce the amount of oral corticosteroids you need while preventing severe asthma attacks and improving your breathing. It is not known if DUPIXENT is safe and effective in children with asthma under 6 years of age. with other medicines for the maintenance treatment of chronic rhinosinusitis with nasal polyps (CRSwNP) in adults and children 12 years of age and older whose disease is not controlled. It is not known if DUPIXENT is safe and effective in children with chronic rhinosinusitis with nasal polyps under 12 years of age. to treat adults and children 1 year of age and older with eosinophilic esophagitis (EoE), who weigh at least 33 pounds (15 kg). It is not known if DUPIXENT is safe and effective in children with eosinophilic esophagitis under 1 year of age, or who weigh less than 33 pounds (15 kg). to treat adults with prurigo nodularis (PN). It is not known if DUPIXENT is safe and effective in children with prurigo nodularis under 18 years of age. with other medicines for the maintenance treatment of adults with inadequately controlled chronic obstructive pulmonary disease (COPD) and a high number of blood eosinophils (a type of white blood cell that may contribute to your COPD). DUPIXENT is used to reduce the number of flare-ups (the worsening of your COPD symptoms for several days) and can improve your breathing. It is not known if DUPIXENT is safe and effective in children with chronic obstructive pulmonary disease under 18 years of age. to treat adults and children 12 years of age and older with chronic spontaneous urticaria (CSU) who continue to have hives that are not controlled with H1 antihistamine treatment. It is not known if DUPIXENT is safe and effective in children with chronic spontaneous urticaria under 12 years of age, or who weigh less than 66 pounds (30 kg).
DUPIXENT is not used to relieve sudden breathing problems and will not replace an inhaled rescue medicine.
DUPIXENT is not used to treat any other forms of hives (urticaria).
IMPORTANT SAFETY INFORMATION
Do not use if you are allergic to dupilumab or to any of the ingredients in DUPIXENT®.
Before using DUPIXENT, tell your healthcare provider about all your medical conditions, including if you:
have eye problems. have a parasitic (helminth) infection. are scheduled to receive any vaccinations. You should not receive a “live vaccine” right before and during treatment with DUPIXENT. are pregnant or plan to become pregnant. It is not known whether DUPIXENT will harm your unborn baby. A pregnancy registry for women who take DUPIXENT during pregnancy collects information about the health of you and your baby. To enroll or get more information call 1-877-311-8972 or go to https://mothertobaby.org/ongoing-study/dupixent/. are breastfeeding or plan to breastfeed. It is not known whether DUPIXENT passes into your breast milk.
A pregnancy registry for women who take DUPIXENT during pregnancy collects information about the health of you and your baby. To enroll or get more information call 1-877-311-8972 or go to https://mothertobaby.org/ongoing-study/dupixent/.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Especially tell your healthcare provider if you are taking oral, topical, or inhaled corticosteroid medicines; have asthma and use an asthma medicine; or have atopic dermatitis, chronic rhinosinusitis with nasal polyps, eosinophilic esophagitis, prurigo nodularis, chronic obstructive pulmonary disease, or chronic spontaneous urticaria, and also have asthma. Do not change or stop your other medicines, including corticosteroid medicine or other asthma medicine, without talking to your healthcare provider. This may cause other symptoms that were controlled by those medicines to come back.
DUPIXENT can cause serious side effects, including:
Allergic reactions. DUPIXENT can cause allergic reactions that can sometimes be severe. Stop using DUPIXENT and tell your healthcare provider or get emergency help right away if you get any of the following signs or symptoms: breathing problems or wheezing, swelling of the face, lips, mouth, tongue or throat, fainting, dizziness, feeling lightheaded, fast pulse, fever, hives, joint pain, general ill feeling, itching, skin rash, swollen lymph nodes, nausea or vomiting, or cramps in your stomach-area. Eye problems. Tell your healthcare provider if you have any new or worsening eye problems, including eye pain or changes in vision, such as blurred vision. Your healthcare provider may send you to an ophthalmologist for an exam if needed Inflammation of your blood vessels. Rarely, this can happen in people with asthma who receive DUPIXENT. This may happen in people who also take a steroid medicine by mouth that is being stopped or the dose is being lowered. Tell your healthcare provider right away if you have: rash, chest pain, worsening shortness of breath, brown or dark colored urine, persistent fever, or a feeling of pins and needles or numbness of your arms or legs. Psoriasis. This can happen in people with atopic dermatitis and asthma who receive DUPIXENT. Tell your healthcare provider about any new skin symptoms. Your healthcare provider may send you to a dermatologist for an examination if needed. Joint aches and pain. Some people who use DUPIXENT have had trouble walking or moving due to their joint symptoms, and in some cases needed to be hospitalized. Tell your healthcare provider about any new or worsening joint symptoms. Your healthcare provider may stop DUPIXENT if you develop joint symptoms.
The most common side effects include:
Eczema: injection site reactions, eye and eyelid inflammation, including redness, swelling, and itching, sometimes with blurred vision, dry eye, cold sores in your mouth or on your lips, and high count of a certain white blood cell (eosinophilia). Asthma: injection site reactions, high count of a certain white blood cell (eosinophilia), pain in the throat (oropharyngeal pain), and parasitic (helminth) infections. Chronic Rhinosinusitis with Nasal Polyps: injection site reactions, eye and eyelid inflammation, including redness, swelling, and itching, sometimes with blurred vision, high count of a certain white blood cell (eosinophilia), gastritis, joint pain (arthralgia), trouble sleeping (insomnia), and toothache. Eosinophilic Esophagitis: injection site reactions, upper respiratory tract infections, cold sores in your mouth or on your lips, and joint pain (arthralgia). Prurigo Nodularis: eye and eyelid inflammation, including redness, swelling, and itching, sometimes with blurred vision, herpes virus infections, common cold symptoms (nasopharyngitis), dizziness, muscle pain, and diarrhea. Chronic Obstructive Pulmonary Disease: injection site reactions, common cold symptoms (nasopharyngitis), high count of a certain white blood cell (eosinophilia), viral infection, back pain, inflammation inside the nose (rhinitis), diarrhea, gastritis, joint pain (arthralgia), toothache, headache, and urinary tract infection. Chronic Spontaneous Urticaria: injection site reactions.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of DUPIXENT. Call your doctor for medical advice about side effects. You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
Use DUPIXENT exactly as prescribed by your healthcare provider. It’s an injection given under the skin (subcutaneous injection). Your healthcare provider will decide if you or your caregiver can inject DUPIXENT. Do not try to prepare and inject DUPIXENT until you or your caregiver have been trained by your healthcare provider. In children 12 years of age and older, it’s recommended DUPIXENT be administered by or under supervision of an adult. In children 6 months to less than 12 years of age, DUPIXENT should be given by a caregiver.
Please see accompanying full Prescribing Information including Patient Information.
About Regeneron Regeneron (NASDAQ: REGN) is a leading biotechnology company that invents, develops and commercializes life-transforming medicines for people with serious diseases. Founded and led by physician-scientists, our unique ability to repeatedly and consistently translate science into medicine has led to numerous approved treatments and product candidates in development, most of which were homegrown in our laboratories. Our medicines and pipeline are designed to help patients with eye diseases, allergic and inflammatory diseases, cancer, cardiovascular and metabolic diseases, neurological diseases, hematologic conditions, infectious diseases, and rare diseases.
Regeneron pushes the boundaries of scientific discovery and accelerates drug development using our proprietary technologies, such as VelociSuite, which produces optimized fully human antibodies and new classes of bispecific antibodies. We are shaping the next frontier of medicine with data-powered insights from the Regeneron Genetics Center® and pioneering genetic medicine platforms, enabling us to identify innovative targets and complementary approaches to potentially treat or cure diseases.
For more information, please visit www.Regeneron.com or follow Regeneron on LinkedIn, Instagram, Facebook or X. Regeneron Forward-Looking Statements and Use of Digital Media This press release includes forward-looking statements that involve risks and uncertainties relating to future events and the future performance of Regeneron Pharmaceuticals, Inc. (“Regeneron” or the “Company”), and actual events or results may differ materially from these forward-looking statements. Words such as “anticipate,” “expect,” “intend,” “plan,” “believe,” “seek,” “estimate,” variations of such words, and similar expressions are intended to identify such forward-looking statements, although not all forward-looking statements contain these identifying words. These statements concern, and these risks and uncertainties include, among others, the nature, timing, and possible success and therapeutic applications of products marketed or otherwise commercialized by Regeneron and/or its collaborators or licensees (collectively, “Regeneron’s Products”) and product candidates being developed by Regeneron and/or its collaborators or licensees (collectively, “Regeneron’s Product Candidates”) and research and clinical programs now underway or planned, including without limitation Dupixent® (dupilumab) for the treatment of chronic obstructive pulmonary disease and asthma as discussed in this press release; uncertainty of the utilization, market acceptance, and commercial success of Regeneron’s Products and Regeneron’s Product Candidates and the impact of studies (whether conducted by Regeneron or others and whether mandated or voluntary), including the studies discussed or referenced in this press release, on any of the foregoing; the likelihood, timing, and scope of possible regulatory approval and commercial launch of Regeneron’s Product Candidates and new indications for Regeneron’s Products, such as Dupixent for the treatment of chronic pruritus of unknown origin, bullous pemphigoid, lichen simplex chronicus, and other potential indications; the ability of Regeneron’s collaborators, licensees, suppliers, or other third parties (as applicable) to perform manufacturing, filling, finishing, packaging, labeling, distribution, and other steps related to Regeneron’s Products and Regeneron’s Product Candidates; the ability of Regeneron to manage supply chains for multiple products and product candidates; safety issues resulting from the administration of Regeneron’s Products (such as Dupixent) and Regeneron’s Product Candidates in patients, including serious complications or side effects in connection with the use of Regeneron’s Products and Regeneron’s Product Candidates in clinical trials; determinations by regulatory and administrative governmental authorities which may delay or restrict Regeneron’s ability to continue to develop or commercialize Regeneron’s Products and Regeneron’s Product Candidates; ongoing regulatory obligations and oversight impacting Regeneron’s Products, research and clinical programs, and business, including those relating to patient privacy; the availability and extent of reimbursement of Regeneron’s Products from third-party payers, including private payer healthcare and insurance programs, health maintenance organizations, pharmacy benefit management companies, and government programs such as Medicare and Medicaid; coverage and reimbursement determinations by such payers and new policies and procedures adopted by such payers; changes in laws, regulations, and policies affecting the healthcare industry; risks associated with tariffs and other trade restrictions; competing drugs and product candidates that may be superior to, or more cost effective than, Regeneron’s Products and Regeneron’s Product Candidates (including biosimilar versions of Regeneron’s Products); the extent to which the results from the research and development programs conducted by Regeneron and/or its collaborators or licensees may be replicated in other studies and/or lead to advancement of product candidates to clinical trials, therapeutic applications, or regulatory approval; unanticipated expenses; the costs of developing, producing, and selling products; the ability of Regeneron to meet any of its financial projections or guidance and changes to the assumptions underlying those projections or guidance; the potential for any license, collaboration, or supply agreement, including Regeneron’s agreements with Sanofi and Bayer (or their respective affiliated companies, as applicable), to be cancelled or terminated; the impact of public health outbreaks, epidemics, or pandemics on Regeneron 's business; and risks associated with litigation and other proceedings and government investigations relating to the Company and/or its operations (including the pending civil proceedings initiated or joined by the U.S. Department of Justice and the U.S. Attorney's Office for the District of Massachusetts ), risks associated with intellectual property of other parties and pending or future litigation relating thereto (including without limitation the patent litigation and other related proceedings relating to EYLEA® (aflibercept) Injection), the ultimate outcome of any such proceedings and investigations, and the impact any of the foregoing may have on Regeneron’s business, prospects, operating results, and financial condition. A more complete description of these and other material risks can be found in Regeneron’s filings with the U.S. Securities and Exchange Commission , including its Form 10-K for the year ended December 31, 2024 and its Form 10-Q for the quarterly period ended March 31, 2025 . Any forward-looking statements are made based on management’s current beliefs and judgment, and the reader is cautioned not to rely on any forward-looking statements made by Regeneron . Regeneron does not undertake any obligation to update (publicly or otherwise) any forward-looking statement, including without limitation any financial projection or guidance, whether as a result of new information, future events, or otherwise.
Regeneron uses its media and investor relations website and social media outlets to publish important information about the Company, including information that may be deemed material to investors. Financial and other information about Regeneron is routinely posted and is accessible on Regeneron 's media and investor relations website (https://investor.regeneron.com) and its LinkedIn page (https://www.linkedin.com/company/regeneron-pharmaceuticals).
_____________________
1 Data on File
Source: Regeneron Pharmaceuticals, Inc.
临床结果临床3期上市批准临床2期
分析
对领域进行一次全面的分析。
登录
或
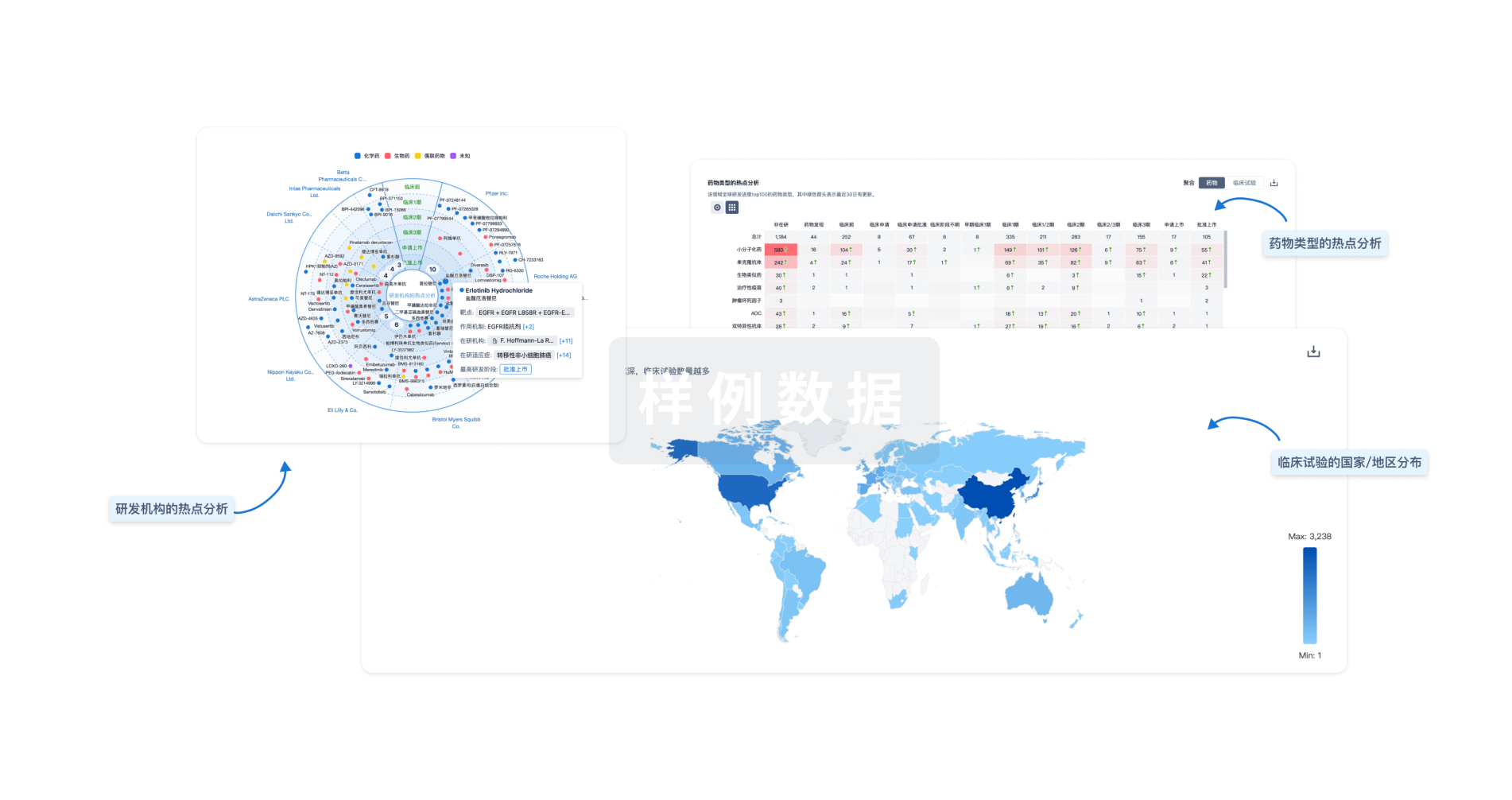
Eureka LS:
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用





