预约演示
更新于:2025-05-07
Acute sinusitis
急性鼻窦炎
更新于:2025-05-07
基本信息
别名 ACUTE SINUSITIS、Acute Sinusitis、Acute infection of nasal sinus, NOS + [26] |
简介 Sinusitis lasting less than or equal to thirty days. |
关联
17
项与 急性鼻窦炎 相关的药物靶点- |
作用机制- |
在研机构 |
原研机构 |
非在研适应症- |
最高研发阶段批准上市 |
首次获批国家/地区 中国 |
首次获批日期2020-08-28 |
靶点- |
作用机制- |
在研机构 |
原研机构- |
非在研适应症- |
最高研发阶段批准上市 |
首次获批国家/地区 澳大利亚 |
首次获批日期2011-12-16 |
173
项与 急性鼻窦炎 相关的临床试验NCT06606873
Anatomical Risk Factors for Orbital Complications in Patients With Acute Rhinosinusitis
Rhinosinusitis is among the most common conditions encountered in the primary care clinic.
开始日期2025-07-01 |
申办/合作机构 |
CTRI/2025/04/084003
Single centric non randomized open clinical trial Phase II in Neer Peenisam with the intervention of siddha medicine Peenisa Thyla Nasiyam (Nasal drops) among patients attending OPD at AAGHIM attached with GSMC chennai - NIL
开始日期2025-04-29 |
申办/合作机构- |
NCT06901297
Multicenter, Double-blind, Placebo-controlled, Randomized, Parallel-group Clinical Trial of the Efficacy and Safety of Raphamin in the Treatment of Acute Rhinosinusitis in Adult Patients
Multicenter double-blind placebo-controlled randomized in parallel groups clinical trial of the efficacy and safety of Raphamin in the treatment of acute rhinosinusitis in adult patients.
开始日期2025-04-03 |
申办/合作机构- |
100 项与 急性鼻窦炎 相关的临床结果
登录后查看更多信息
100 项与 急性鼻窦炎 相关的转化医学
登录后查看更多信息
0 项与 急性鼻窦炎 相关的专利(医药)
登录后查看更多信息
2,170
项与 急性鼻窦炎 相关的文献(医药)2025-12-01·Current Allergy and Asthma Reports
Herbal Medicine in Acute and Chronic Sinusitis; Still a Cinderella?
Review
作者: Liva, Georgia ; Prokopakis, Emmanuel ; Vardouniotis, Alexios ; Doulaptsi, Maria ; Karatzanis, Alexander ; Vlastos, Ioannis
2025-04-01·European Archives of Oto-Rhino-Laryngology
Management of intracranial and orbital complications of acute rhinosinusitis and acute otitis media in the post covid-19 era in pediatric patients.
Article
作者: Speranzon, Luca ; Signorelli, Francesco ; Salonna, Francesco ; Caselli, Desiree ; Quaranta, Nicola ; De Giglio, Vito ; Pontillo, Vito ; Messina, Raffaella ; Foscolo, Valentina
2025-04-01·Journal of Breath Research
Xylometazoline-induced change in aspirated nasal nitric oxide detects obstructed paranasal ostia
Article
作者: Numminen, Jura ; Kivekäs, Ilkka ; Rautiainen, Markus ; Lehtimäki, Lauri ; Tamminen, Pekka ; Järnstedt, Jorma
42
项与 急性鼻窦炎 相关的新闻(医药)2025-04-18
Sanofi’s respiratory pipeline advances with new data in asthma and plans for new clinical studies in COPD
New phase 2 data for amlitelimab show efficacy in heterogeneous inflammatory asthma
Lunsekimig now targeting chronic rhinosinusitis and COPD in addition to asthma
Itepekimab expanding into chronic rhinosinusitis along with COPD and bronchiectasis; phase 3 readouts in COPD in H2 2025 and bronchiectasis in 2026
April 15, 2025, Sanofi today shared new progress from its mid- to late-stage respiratory pipeline, including preliminary phase 2 results for amlitelimab in adults with moderate-to-severe asthma.
Preliminary results from the TIDE-Asthma phase 2 study (clinical study identifier: NCT05421598) show that the primary endpoint of annualized exacerbation rate at week 48 was not met at the highest dose level, leading to nominal significance at the medium and low doses. However, the study demonstrates amlitelimab's compelling efficacy in heterogeneous inflammatory asthma, potentially representing a breakthrough for this underserved patient population if observed in later studies. Treatment with amlitelimab led to nominally significant and clinically meaningful reductions in asthma exacerbations at the medium dose tested and a numerically greater reduction in exacerbations at the high dose at week 60. The study also demonstrated nominally significant and clinically meaningful improvement in secondary endpoints of lung function and asthma control. Notably, in a patient sub-group defined by biomarkers (eosinophils ≥300 cells/ml and elevated neutrophils), amlitelimab showed nominally significant and clinically meaningful improvements in exacerbations (with a reduction of more than 70%), lung function and asthma control at week 60. These results demonstrate that amlitelimab has potential to improve key disease outcomes in asthma patients with continued unmet need. The phase 3 program is currently being planned.
Executive Vice President, Head of Research & Development
“We are pleased by the significant progress we have made with our pipeline across respiratory indications. In asthma, amlitelimab shows potential as an effective, long-acting medicine, including in patients with moderate-to-severe heterogenous inflammation. If the preliminary effect we have seen is confirmed in phase 3 studies, amlitelimab could become a differentiated treatment option in asthma. These data validate our strategy to advance innovative science and provide new solutions for patients with challenging-to-treat respiratory diseases.”
Amlitelimab has a unique non-depleting mechanism of action targeting OX40-Ligand with the potential to durably restore immune balance, with a sustained effect and infrequent dosing. In the TIDE-Asthma study, patients were treated every four weeks for the first 24 weeks and every 12 weeks for the remaining 36 weeks. The durable efficacy shown by amlitelimab through 60 weeks of treatment supports a quarterly maintenance dosing schedule. The safety profile was consistent with previous studies across indications, with no new safety signals identified throughout the 60-week treatment period. The incidence of treatment emergent adverse effects (TEAEs) or treatment discontinuation was similar between the amlitelimab and the placebo groups. The most frequent TEAEs (≥ 5% in any arm) more common (≥ 1%) than placebo were COVID-19, bronchitis, acute sinusitis and headache. All were mild-to-moderate in severity and all non-serious.
Lunsekimig: potential for broader use in chronic obstructive pulmonary disease (COPD)
Lunsekimig is being explored in a broad population of asthma patients, regardless of their inflammation and severity status.
The readout of the AIRCULES phase 2 study (clinical study identifier: NCT06102005) in moderate to severe asthma is anticipated in 2026 while the AIRLYMPUS phase 2 study (clinical study identifier: NCT06676319) in high-risk asthma was initiated in Q4 2024.
The readout of the phase 2 study in patients with chronic rhinosinusitis with nasal polyps (clinical study identifier: NCT06454240) is anticipated in 2026.
A phase 2/3 study in COPD is anticipated to begin in 2025.
Itepekimab: expanding clinical studies beyond COPD into chronic rhinosinusitis
In partnership with Regeneron, two phase 3 studies in patients with chronic rhinosinusitis with nasal polyps (CRSwNP), CEREN 1 (clinical study identifier: NCT06834347) and CEREN 2 (clinical study identifier: NCT06834360) and one phase 2 study in patients with chronic rhinosinusitis without nasal polyps (CRSsNP) (clinical study identifier: NCT06691113) were initiated in Q1 2025.
Itepekimab is currently being explored in patients with COPD in two phase 3 studies AERIFY-1 (clinical study identifier: NCT04701983) and AERIFY-2 (clinical study identifier: NCT04751487) with the readout anticipated in H2 2025, and in one phase 2 study, AERIFY-3 (clinical study identifier: NCT05326412), with the readout anticipated in H2 2025.
Finally, itepekimab is being explored in a phase 2 study (clinical study identifier: NCT06280391) in bronchiectasis, with the readout anticipated in 2026.
Amlitelimab is a fully human non-T cell depleting monoclonal antibody that blocks OX40-Ligand, a key immune regulator, and has the potential to be a first- or best-in-class treatment for a range of immune-mediated diseases and inflammatory disorders, including moderate-to-severe atopic dermatitis (phase 3), asthma (phase 2), hidradenitis suppurativa (phase 2), systemic sclerosis (phase 2), celiac disease (phase 2), and alopecia (phase 2). By targeting OX40-Ligand, amlitelimab aims to preserve the balance between pro-inflammatory and regulatory T cells. Amlitelimab is currently under clinical investigation, and its safety and efficacy have not been evaluated by any regulatory authority.
The phase 2 TIDE-Asthma study is a randomized, double-blind, placebo-controlled, dose-ranging study, evaluating amlitelimab as an add-on therapy in 437 adults with moderate-to-severe asthma. Participants were on standard-of-care medicines with medium-to-high doses of inhaled corticosteroids and up to two other controllers. The study included three dose levels of amlitelimab, each with a loading dose administered every four weeks for the first 24 weeks, followed by once every 12 weeks until week 60, with participants randomized 2:1:2:2 to receive one of the three active doses or placebo. The primary endpoint was the annualized rate of severe asthma exacerbations. Key secondary endpoints include lung function (pre-BD FEV1) and asthma control (ACQ-5).
Lunsekimig is a novel Nanobody VHH® that combines targeting of IL13, a downstream cytokine causing tissue organ damage in respiratory diseases and TSLP, an upstream initiator of inflammation. Pre-clinical research suggests that the combination of these targets simultaneously can potentially lead to additive and synergistic benefits in immune-mediated diseases such as asthma. Lunsekimig is currently under clinical investigation, and its safety and efficacy have not been evaluated by any regulatory authority.
In partnership with Regeneron, Sanofi is exploring itepekimab, a fully human monoclonal antibody that binds to and inhibits IL33, an initiator and amplifier of broad inflammation in respiratory diseases. Itepekimab is currently under clinical investigation, and its safety and efficacy have not been evaluated by any regulatory authority.
We are an innovative global healthcare company, driven by one purpose: we chase the miracles of science to improve people’s lives. Our team, across the world, is dedicated to transforming the practice of medicine by working to turn the impossible into the possible. We provide potentially life-changing treatment options and life-saving vaccine protection to millions of people globally, while putting sustainability and social responsibility at the center of our ambitions.
The content above comes from the network. if any infringement, please contact us to modify.
临床结果
2025-04-15
PARIS, France I April 15, 2025 I
Sanofi today shared new progress from its mid- to late-stage respiratory pipeline, including preliminary phase 2 results for amlitelimab in adults with moderate-to-severe asthma.
Amlitelimab: clinically meaningful efficacy in asthma
Preliminary results from the TIDE-Asthma phase 2 study (clinical study identifier: NCT05421598) show that the primary endpoint of annualized exacerbation rate at week 48 was not met at the highest dose level, leading to nominal significance at the medium and low doses. However, the study demonstrates amlitelimab’s compelling efficacy in heterogeneous inflammatory asthma, potentially representing a breakthrough for this underserved patient population if observed in later studies. Treatment with amlitelimab led to nominally significant and clinically meaningful reductions in asthma exacerbations at the medium dose tested and a numerically greater reduction in exacerbations at the high dose at week 60. The study also demonstrated nominally significant and clinically meaningful improvement in secondary endpoints of lung function and asthma control. Notably, in a patient sub-group defined by biomarkers (eosinophils ≥300 cells/ml and elevated neutrophils), amlitelimab showed nominally significant and clinically meaningful improvements in exacerbations (with a reduction of more than 70%), lung function and asthma control at week 60. These results demonstrate that amlitelimab has potential to improve key disease outcomes in asthma patients with continued unmet need. The phase 3 program is currently being planned.
Houman Ashrafian
Executive Vice President, Head of Research & Development
“We are pleased by the significant progress we have made with our pipeline across respiratory indications. In asthma, amlitelimab shows potential as an effective, long-acting medicine, including in patients with moderate-to-severe heterogenous inflammation. If the preliminary effect we have seen is confirmed in phase 3 studies, amlitelimab could become a differentiated treatment option in asthma. These data validate our strategy to advance innovative science and provide new solutions for patients with challenging-to-treat respiratory diseases.”
Amlitelimab has a unique non-depleting mechanism of action targeting OX40-Ligand with the potential to durably restore immune balance, with a sustained effect and infrequent dosing. In the TIDE-Asthma study, patients were treated every four weeks for the first 24 weeks and every 12 weeks for the remaining 36 weeks. The durable efficacy shown by amlitelimab through 60 weeks of treatment supports a quarterly maintenance dosing schedule. The safety profile was consistent with previous studies across indications, with no new safety signals identified throughout the 60-week treatment period. The incidence of treatment emergent adverse effects (TEAEs) or treatment discontinuation was similar between the amlitelimab and the placebo groups. The most frequent TEAEs (≥ 5% in any arm) more common (≥ 1%) than placebo were COVID-19, bronchitis, acute sinusitis and headache. All were mild-to-moderate in severity and all non-serious.
Full and final results will be presented at a forthcoming medical meeting.
Lunsekimig: potential for broader use in chronic obstructive pulmonary disease
(COPD)
Lunsekimig is being explored in a broad population of asthma patients, regardless of their inflammation and severity status.
The readout of the AIRCULES phase 2 study (clinical study identifier: NCT06102005) in moderate to severe asthma is anticipated in 2026 while the AIRLYMPUS phase 2 study (clinical study identifier: NCT06676319) in high-risk asthma was initiated in Q4 2024.
The readout of the phase 2 study in patients with chronic rhinosinusitis with nasal polyps (clinical study identifier: NCT06454240) is anticipated in 2026.
A phase 2/3 study in COPD is anticipated to begin in 2025.
Itepekimab: expanding clinical studies beyond COPD into chronic rhinosinusitis
In partnership with Regeneron, two phase 3 studies in patients with chronic rhinosinusitis with nasal polyps (CRSwNP), CEREN 1 (clinical study identifier: NCT06834347) and CEREN 2 (clinical study identifier: NCT06834360) and one phase 2 study in patients with chronic rhinosinusitis without nasal polyps (CRSsNP) (clinical study identifier: NCT06691113) were initiated in Q1 2025.
Itepekimab is currently being explored in patients with COPD in two phase 3 studies AERIFY-1 (clinical study identifier: NCT04701983) and AERIFY-2 (clinical study identifier: NCT04751487) with the readout anticipated in H2 2025, and in one phase 2 study, AERIFY-3 (clinical study identifier: NCT05326412), with the readout anticipated in H2 2025.
Finally, itepekimab is being explored in a phase 2 study (clinical study identifier: NCT06280391) in bronchiectasis, with the readout anticipated in 2026.
About amlitelimab
Amlitelimab is a fully human non-T cell depleting monoclonal antibody that blocks OX40-Ligand, a key immune regulator, and has the potential to be a first- or best-in-class treatment for a range of immune-mediated diseases and inflammatory disorders, including moderate-to-severe atopic dermatitis (phase 3), asthma (phase 2), hidradenitis suppurativa (phase 2), systemic sclerosis (phase 2), celiac disease (phase 2), and alopecia (phase 2). By targeting OX40-Ligand, amlitelimab aims to preserve the balance between pro-inflammatory and regulatory T cells. Amlitelimab is currently under clinical investigation, and its safety and efficacy have not been evaluated by any regulatory authority.
About TIDE-Asthma
The phase 2 TIDE-Asthma study is a randomized, double-blind, placebo-controlled, dose-ranging study, evaluating amlitelimab as an add-on therapy in 437 adults with moderate-to-severe asthma. Participants were on standard-of-care medicines with medium-to-high doses of inhaled corticosteroids and up to two other controllers. The study included three dose levels of amlitelimab, each with a loading dose administered every four weeks for the first 24 weeks, followed by once every 12 weeks until week 60, with participants randomized 2:1:2:2 to receive one of the three active doses or placebo. The primary endpoint was the annualized rate of severe asthma exacerbations. Key secondary endpoints include lung function (pre-BD FEV1) and asthma control (ACQ-5).
About lunsekimig
Lunsekimig is a novel Nanobody VHH
®
that combines targeting of IL13, a downstream cytokine causing tissue organ damage in respiratory diseases and TSLP, an upstream initiator of inflammation. Pre-clinical research suggests that the combination of these targets simultaneously can potentially lead to additive and synergistic benefits in immune-mediated diseases such as asthma. Lunsekimig is currently under clinical investigation, and its safety and efficacy have not been evaluated by any regulatory authority.
About itepekimab
In partnership with Regeneron, Sanofi is exploring itepekimab, a fully human monoclonal antibody that binds to and inhibits IL33, an initiator and amplifier of broad inflammation in respiratory diseases. Itepekimab is currently under clinical investigation, and its safety and efficacy have not been evaluated by any regulatory authority.
About Sanofi
We are an innovative global healthcare company, driven by one purpose: we chase the miracles of science to improve people’s lives. Our team, across the world, is dedicated to transforming the practice of medicine by working to turn the impossible into the possible. We provide potentially life-changing treatment options and life-saving vaccine protection to millions of people globally, while putting sustainability and social responsibility at the center of our ambitions.
SOURCE:
Sanofi
临床2期临床结果临床3期
2025-03-26
Company reports fourth quarter and full year 2024 XHANCE net revenue of $22.4 million and $78.2 million, increases of 13% and 10% compared to prior year periods Company reports 23% prescription growth from third quarter 2024 to fourth quarter 2024 YARDLEY, Pa., March 26, 2025 (GLOBE NEWSWIRE) -- Optinose (NASDAQ:OPTN), a pharmaceutical company focused on patients treated by ear, nose and throat (ENT) and allergy specialists, today reported financial results for the quarter and year ended December 31, 2024, and provided recent operational highlights. Fourth Quarter 2024 and Recent Highlights New Prescriptions (NRx) and Total Prescriptions (TRx)An inflection in prescription demand first observed in September 2024 NRx continued in fourth quarter 2024 and extended to TRx. NRx increased 12% from approximately 25,600 in third quarter 2024 to approximately 28,700 in fourth quarter 2024. TRx increased 23% from approximately 63,900 in third quarter 2024 to approximately 78,500 in fourth quarter 2024. Fourth Quarter 2024 Financial Results RevenuesThe Company reported $22.4 million in net revenue from sales of XHANCE during the three-month period ended December 31, 2024, an increase of 13% compared to $19.9 million during the three-month period ended December 31, 2023. For the twelve-month period ended December 31, 2024, the Company reported $78.2 million in net revenue from sales of XHANCE, an increase of 10% compared to $71.0 million during the twelve-month period ended December 31, 2023. Costs and expenses and net lossFor the three-month and twelve-month periods ended December 31, 2024, research and development expenses were $0.8 million and $3.9 million, respectively. Selling, general and administrative expenses were $19.3 million and $83.5 million during the three-month and twelve-month periods ended December 31, 2024, respectively. In total, SG&A plus R&D expenses increased by $2.2 million, to $87.3 million for the twelve-month period ended December 31, 2024 when compared to the twelve-month period ended December 31, 2023 total of $85.1 million. Income from operations was for the three-month period ended December 31, 2024, was $0.4 million. This is the first three-month period in which the Company reported income from operations. The net loss for the three-month period ended December 31, 2024 was $0.4 million, or $0.04 per share (basic and diluted). The net loss for the twelve-month period ended December 31, 2024 was $21.5 million, or $2.12 per share (basic and diluted). Balance SheetThe Company had cash and cash equivalents of $84.5 million as of December 31, 2024. OptiNose, Inc.Condensed Consolidated Statement of Operations(in thousands, except share and per share data)(Unaudited)
Three Months Ended Year Ended December 31, December 31, 2024 2023 2024 2023 Revenues:
Net product revenues $22,418 $19,865 $78,226 $70,987 Total revenues 22,418 19,865 78,226 70,987 Costs and expenses:
Cost of product sales $1,954 $2,131 $7,231 $8,633 Research and development 772 1,286 3,855 5,303 Selling, general and administrative 19,338 18,960 83,459 79,799 Total costs and expenses 22,064 22,377 94,545 93,735 Income (loss) from operations 354 (2,512) (16,319) (22,748)Other (income) expense 714 7,455 5,222 12,735 Net (loss) $(360) $(9,967) $(21,541) $(35,483)Net loss per share of common stock, basic and diluted $(0.03) $(1.33) $(2.12) $(4.75)Weighted average common shares outstanding, basic and diluted 11,635,909 7,487,465 10,156,745 7,472,035 OptiNose, Inc.Condensed Consolidated Balance Sheet Data(in thousands)
December 31 December 31, 2024 2023
Cash and cash equivalents $84,485 $73,684 Other assets 44,300 34,045 Total assets $128,785 $107,729
Total current liabilities (1) $162,813 $176,524 Other liabilities 6,332 17,811 Total stockholders' deficit (40,360) (86,606)Total liabilities and stockholders' deficit $128,785 $107,729
(1) – All outstanding principal and fees payable upon maturity have been classified as a current liability in accordance with Generally Accepted Accounting Principles ("GAAP") because, as of the date hereof, the Company believes that it is probable that it will not maintain compliance with certain financial covenants contained in its Amended and Restated Note Purchase Agreement for at least the next 12-months. As a result, the Company's audited financial statements for the year ended December 31, 2024 (“2024 Audited Financial Statements”) will state that there is substantial doubt about the Company's ability to continue as a going concern (i.e., a "going concern" paragraph). Please refer to the Company’s Annual Report on Form 10-K for the year ended December 31, 2024 (including the 2024 Audited Financial Statements) which will be filed after the issuance of this press release for additional information. About OptinoseOptinose is a specialty pharmaceutical company focused on serving the needs of patients cared for by ear, nose and throat (ENT) and allergy specialists. To learn more, please visit www.optinose.com or follow us on Twitter and LinkedIn. About XHANCEXHANCE is a drug-device combination product that uses the Exhalation Delivery System™ (also referred to as the EDS®) designed to deliver a topical steroid to the high and deep regions of the nasal cavity where sinuses ventilate and drain. XHANCE is approved by the U.S. Food and Drug Administration for both the treatment of chronic rhinosinusitis without nasal polyps (also called chronic sinusitis) and chronic rhinosinusitis with nasal polyps (also called nasal polyps) in patients 18 years of age or older. IMPORTANT SAFETY INFORMATION CONTRAINDICATIONS: Hypersensitivity to any ingredient in XHANCE. WARNINGS AND PRECAUTIONS: Local nasal adverse reactions, including epistaxis, erosion, ulceration, septal perforation, Candida albicans infection, and impaired wound healing, can occur. Monitor patients periodically for signs of possible changes on the nasal mucosa. Avoid use in patients with recent nasal ulcerations, nasal surgery, or nasal trauma until healing has occurred.Glaucoma and cataracts may occur with long-term use. Consider referral to an ophthalmologist in patients who develop ocular symptoms or use XHANCE long-term.Hypersensitivity reactions (e.g., anaphylaxis, angioedema, urticaria, contact dermatitis, rash, hypotension, and bronchospasm) have been reported after administration of fluticasone propionate. Discontinue XHANCE if such reactions occur.Immunosuppression and infections can occur, including potential increased susceptibility to or worsening of infections (e.g., existing tuberculosis; fungal, bacterial, viral, or parasitic infection; ocular herpes simplex). Use with caution in patients with these infections. More serious or even fatal course of chickenpox or measles can occur in susceptible patients.Hypercorticism and adrenal suppression may occur with very high dosages or at the regular dosage in susceptible individuals. If such changes occur, discontinue XHANCE slowly.Assess for decrease in bone mineral density initially and periodically thereafter. ADVERSE REACTIONS: Chronic rhinosinusitis without nasal polyps: The most common adverse reactions (incidence ≥3%) are epistaxis, headache, and nasopharyngitis.Chronic rhinosinusitis with nasal polyps: The most common adverse reactions (incidence ≥3%) are epistaxis, nasal septal ulceration, nasopharyngitis, nasal mucosal erythema, nasal mucosal ulcerations, nasal congestion, acute sinusitis, nasal septal erythema, headache, and pharyngitis. DRUG INTERACTIONS: Strong cytochrome P450 3A4 inhibitors (e.g., ritonavir, ketoconazole): Use not recommended. May increase risk of systemic corticosteroid effects. USE IN SPECIFIC POPULATIONS: Hepatic impairment. Monitor patients for signs of increased drug exposure.Please see full Prescribing Information, including Instructions for Use Cautionary Note on Forward-Looking StatementsThis press release contains forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. All statements that are not historical facts are hereby identified as forward-looking statements for this purpose and include, among others, statements relating to the potential benefits of XHANCE; the potential benefits of the Exhalation Delivery System; the Company's belief that it is probable that it will not maintain compliance with certain financial covenants contained in its Amended and Restated Note Purchase Agreement for at least the next 12-months and the consequences thereof; and other statements regarding the Company's future operations, financial performance, financial position, prospects, objectives, strategies and other future events. Forward-looking statements are based upon management’s current expectations and assumptions and are subject to a number of risks, uncertainties and other factors that could cause actual results and events to differ materially and adversely from those indicated by such forward-looking statements including, among others: physician and patient acceptance of XHANCE for its new indication; the Company’s ability to maintain adequate third-party reimbursement for XHANCE (including its new indication); the prevalence of chronic sinusitis and market opportunities for XHANCE may be smaller than expected; the Company’s ability to efficiently generate XHANCE prescriptions and net revenues; unanticipated costs and expenses; the risk that the positive inflection in new XHANCE prescriptions starting in September does not continue and grow; the Company’s ability to comply with the covenants and other terms of its Amended and Restated Note Purchase Agreement; the Company’s ability to continue as a going concern; risks and uncertainties relating to intellectual property and competitive products; and the risks, uncertainties and other factors discussed under the caption "Item 1A. Risk Factors" and elsewhere in the Company’s most recent Form 10-K and Form 10-Q filings with the Securities and Exchange Commission – which are available at www.sec.gov. As a result, you are cautioned not to place undue reliance on any forward-looking statements. Any forward-looking statements made in this press release speak only as of the date of this press release, and the Company undertakes no obligation to update such forward-looking statements, whether as a result of new information, future developments or otherwise. Optinose Investor Contact Jonathan Neely jonathan.neely@optinose.com267.521.0531
财报上市批准
分析
对领域进行一次全面的分析。
登录
或
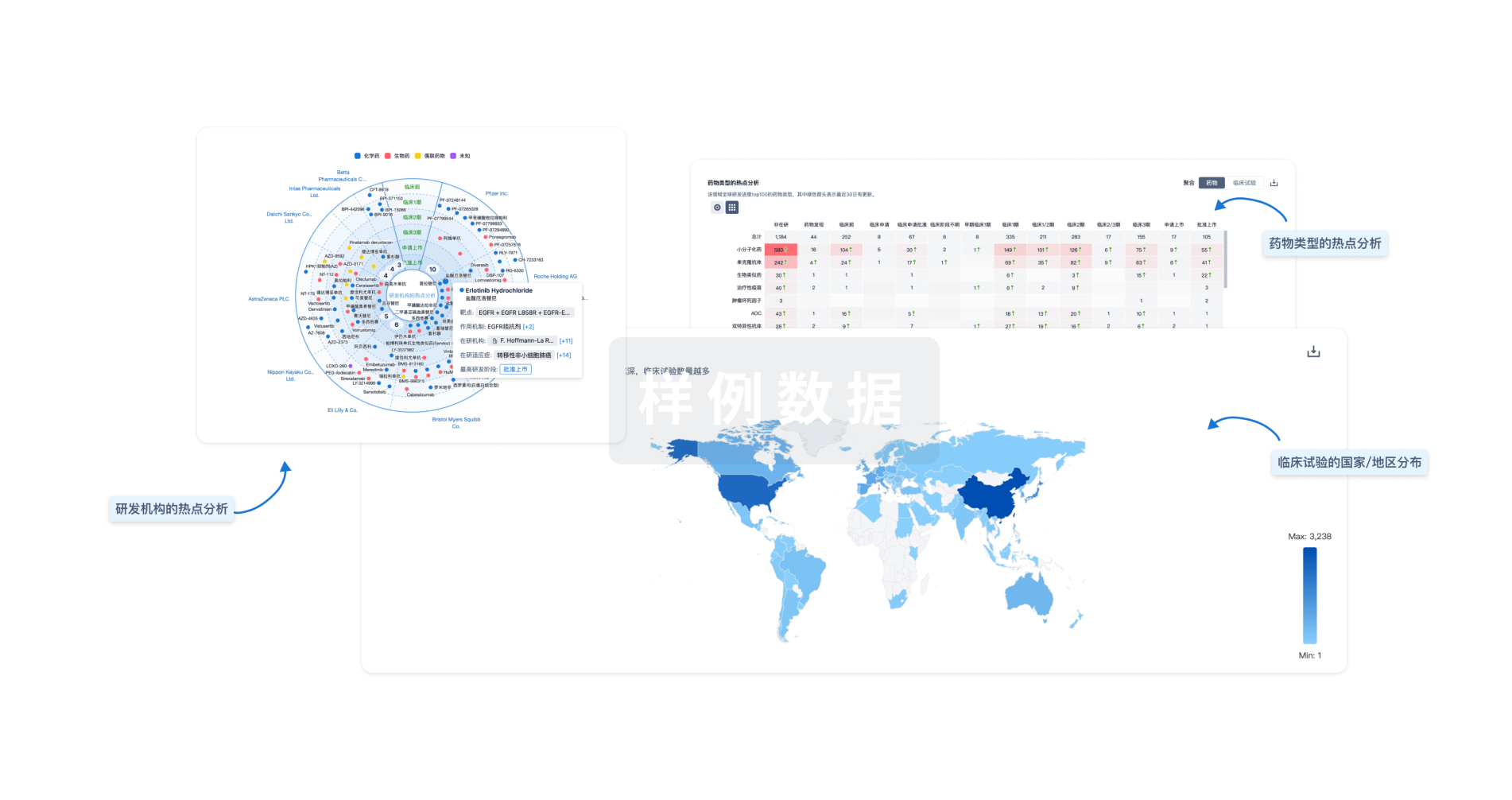
Eureka LS:
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用



