预约演示
更新于:2025-05-07
Intermittent asthma
间歇性哮喘
更新于:2025-05-07
基本信息
别名 Intermittent asthma、Intermittent asthma (disorder)、intermittent asthma + [1] |
简介- |
关联
13
项与 间歇性哮喘 相关的药物作用机制 β2-adrenergic receptor激动剂 |
最高研发阶段批准上市 |
首次获批国家/地区 欧洲 |
首次获批日期2013-12-20 |
作用机制 GR激动剂 [+1] |
原研机构 |
最高研发阶段批准上市 |
首次获批国家/地区 澳大利亚 |
首次获批日期2002-05-27 |
作用机制 H1 receptor拮抗剂 |
最高研发阶段批准上市 |
首次获批国家/地区 欧盟 [+3] |
首次获批日期2001-01-15 |
60
项与 间歇性哮喘 相关的临床试验NCT06849245
Measuring Whether Promotion of a Digital Social Intervention by Primary Care Healthcare Professionals and Subsequent Engagement With Online Peer Support Improves Health and Well-being of Patients With Asthma and is Cost-effective: a Randomised Controlled Trial
The goal of this study is to deliver a definitive randomised controlled trial to measure the effectiveness and assess cost-effectiveness of a digital social intervention for patients with asthma, composed of promoting engagement with online peer support in a primary care consultation, followed by engagement with online peer support for 12 months. The main questions/objectives this study aims to answer/address are:
* Does promoting engagement with an online health community in primary care help people with troublesome asthma to experience fewer asthma symptoms?
* To assess cost-effectiveness of the intervention (including quality of life, well-being, use of healthcare services etc); stakeholder satisfaction (patients and healthcare professionals) with the intervention; fidelity of protocol delivery; context in which positive outcomes can be triggered.
* Does promoting engagement with an online health community in primary care help people with troublesome asthma to experience fewer asthma symptoms?
* To assess cost-effectiveness of the intervention (including quality of life, well-being, use of healthcare services etc); stakeholder satisfaction (patients and healthcare professionals) with the intervention; fidelity of protocol delivery; context in which positive outcomes can be triggered.
开始日期2025-05-01 |
申办/合作机构 |
CTRI/2023/11/059643
Development and validation of a novel tool to measuremedication adherence for select NCDs in the Indian population -An Exploratory sequential mixed method multicentric study - JIMAT
开始日期2024-08-01 |
CTRI/2024/02/063365
Clinical validation of Unani pharmacopoeial formulation Laooq Zeequnnafas Balghami in Zeeq un Nafas (Bronchial asthma) - NIL
开始日期2024-03-05 |
申办/合作机构- |
100 项与 间歇性哮喘 相关的临床结果
登录后查看更多信息
100 项与 间歇性哮喘 相关的转化医学
登录后查看更多信息
0 项与 间歇性哮喘 相关的专利(医药)
登录后查看更多信息
492
项与 间歇性哮喘 相关的文献(医药)2025-03-01·Annals of Allergy, Asthma & Immunology
Mild asthma—What matters to patients and parents
Article
作者: Freigeh, George E ; Mohan, Arjun ; Wettenstein, Rachel P ; Nelson, Belinda ; Greimann, Emma ; Baptist, Alan ; Carpenter, Laurie M
2025-02-01·World Allergy Organization Journal
Development and clinical assessment of a novel probiotic candy in the prevention of respiratory infections in asthmatic children
Article
作者: Ahanchian, Hamid ; Moazzen, Nasrin ; Kianifar, Hamidreza ; Sly, Peter D ; Pahlevanloo, Abolfazl ; Sadeghi, Tahereh ; Jafari, Seyed Ali ; Tafrishi, Rana ; Kiani, Mohammadali
2025-01-14·Blood Advances
Respiratory phenotype and health care utilization patterns by adults with sickle cell disease
Article
作者: Lin, Michelle P. ; Nortey, Nii ; Agawu, Atu ; Zebrowski, Alexis ; Glassberg, Jeffrey ; Jacobs, Charleen
1
项与 间歇性哮喘 相关的新闻(医药)2022-11-23
A new study suggests individuals with persistent asthma have higher levels of inflammation and artery plaque, which may increase heart attack or stroke risk. An analysis of data for more than 5,000 adults has found that those with persistent asthma appeared to be at increased risk of heart attack or stroke. Individuals with persistent asthma had nearly twice the odds of having plaque in their carotid arteries as those without asthma, after adjusting for age, sex, race/ethnicity, weight, other health conditions, prescription medication use and smoking. Study participants with persistent asthma also had higher levels of inflammatory biomarkers as compared to those without asthma.
Adults with persistent asthma may be at increased risk of heart attack or stroke because of excess plaque buildup in the carotid arteries, according to new research published today in the Journal of the American Heart Association, an open access, peer-reviewed journal of the American Heart Association. People in the study had more plaque buildup in the carotid arteries, large arteries on the left and right side of the neck that carry blood to the brain, compared to people without asthma.
Asthma is a respiratory condition that causes a person's airways to become inflamed -- often due to allergic reactions -- making it difficult to breathe. Chronic inflammation over time is known to contribute to artery plaque buildup known as atherosclerosis and is associated with a higher risk of plaques rupturing, triggering a heart attack or stroke.
"Many physicians and patients don't realize that asthmatic airway inflammation may affect the arteries, so for people with persistent asthma, addressing risk factors for cardiovascular disease may be really helpful," said lead study author Matthew C. Tattersall, D.O., M.S., assistant professor in the department of medicine at the University of Wisconsin in Madison. "The presence and burden of carotid artery plaque is a strong predictor of future cardiovascular events."
For this analysis, researchers used data from participants enrolled in the Multi-Ethnic Study of Atherosclerosis (MESA) study to examine the potential association between asthma and carotid artery plaque. MESA is a research study of nearly 7,000 adults that began in 2000 and is still following participants today in six locations across the United States: Baltimore; Chicago; New York; Los Angeles County, California; Forsyth County, North Carolina; and St. Paul, Minnesota. At the time of enrollment, all participants in MESA were free from cardiovascular disease.
The researchers reviewed health data for 5,029 adults, average age of 61, who had baseline risk factors for cardiovascular disease and for whom there was carotid ultrasound data. The participant group is diverse: 26% of adults self-identified as African American, 23% self-identified as Hispanic and 12% self-identified as Chinese. Additionally, more than half of the group (53%) was female.
The participants in this analysis's cohort were categorized as having persistent asthma, intermittent asthma or not having asthma. The subgroup with persistent asthma, defined as using daily controller medications to control asthma symptoms, consisted of 109 participants; the subgroup with intermittent asthma, defined as a history of asthma, but not using daily medications to control asthma symptoms, consisted of 388 participants; and the remaining participants did not have asthma.
At the start of the MESA study, all participants had an ultrasound of the left and right carotid arteries to identify any carotid artery plaque. The total plaque score defined the number of plaques in the walls of both carotid arteries. Blood levels of inflammatory biomarkers interleukin-6 (IL-6) and C-reactive protein (CRP) were also measured at the start of the MESA study.
The analysis found:
When compared to participants without asthma, those with persistent asthma had higher levels of inflammatory biomarkers. (Individuals with persistent asthma had an average IL-6 level of 1.89 pg/mL, while those free from asthma had an average IL-6 level of 1.52 pg/mL.) The researchers found that accounting for IL-6 and CRP in the fully adjusted analysis did not reduce the association between persistent asthma and carotid artery plaque.
"This analysis tells us that the increased risk for carotid plaques among people with persistent asthma is probably affected by multiple factors," Tattersall said. "Participants who have persistent asthma had elevated levels of inflammation in their blood, even though their asthma was treated with medication, which highlights the inflammatory features of asthma. We know that higher levels of inflammation lead to negative effects on the cardiovascular system."
In 2019, the American Heart Association released guidelines for primary prevention of cardiovascular disease included inflammatory disorders such as arthritis and lupus as cardiovascular risk-enhancing factors. This study adds to the understanding of the impact of inflammatory diseases on cardiovascular health.
"The most important message from our findings is that more significant forms of asthma are associated with more cardiovascular disease and cardiovascular events," Tattersall said. "Addressing cardiovascular risk factors through lifestyle and behavior adjustments can be a powerful preventive tool for patients with more severe forms of asthma."
Everyone can improve their cardiovascular health by following the American Heart Association's Life's Essential 8: eating healthy food, being physically active, not smoking, getting enough sleep, maintaining a healthy weight, and controlling cholesterol, blood sugar and blood pressure levels. Cardiovascular disease claims more lives each year in the U.S. than all forms of cancer and chronic lower respiratory disease combined, according to the American Heart Association.
The study's main limitation is that it was observational since it is analysis of data, therefore, the findings indicate an association between asthma and increased cardiovascular disease risk, not cause and effect.
Co-authors are Alison S. Dasiewicz, M.S.; Robyn L. McClelland, Ph.D.; Nizar N. Jarjour, M.D.; Claudia E. Korcarz, D.V.M.; Carol C. Mitchell, Ph.D.; Stephane Esnault, Ph.D.; Moyses Szklo, M.D., M.P.H.; and James H. Stein, M.D., FAHA.
The study was supported by an American Heart Association Peer Development Award.
AHA会议临床研究
分析
对领域进行一次全面的分析。
登录
或
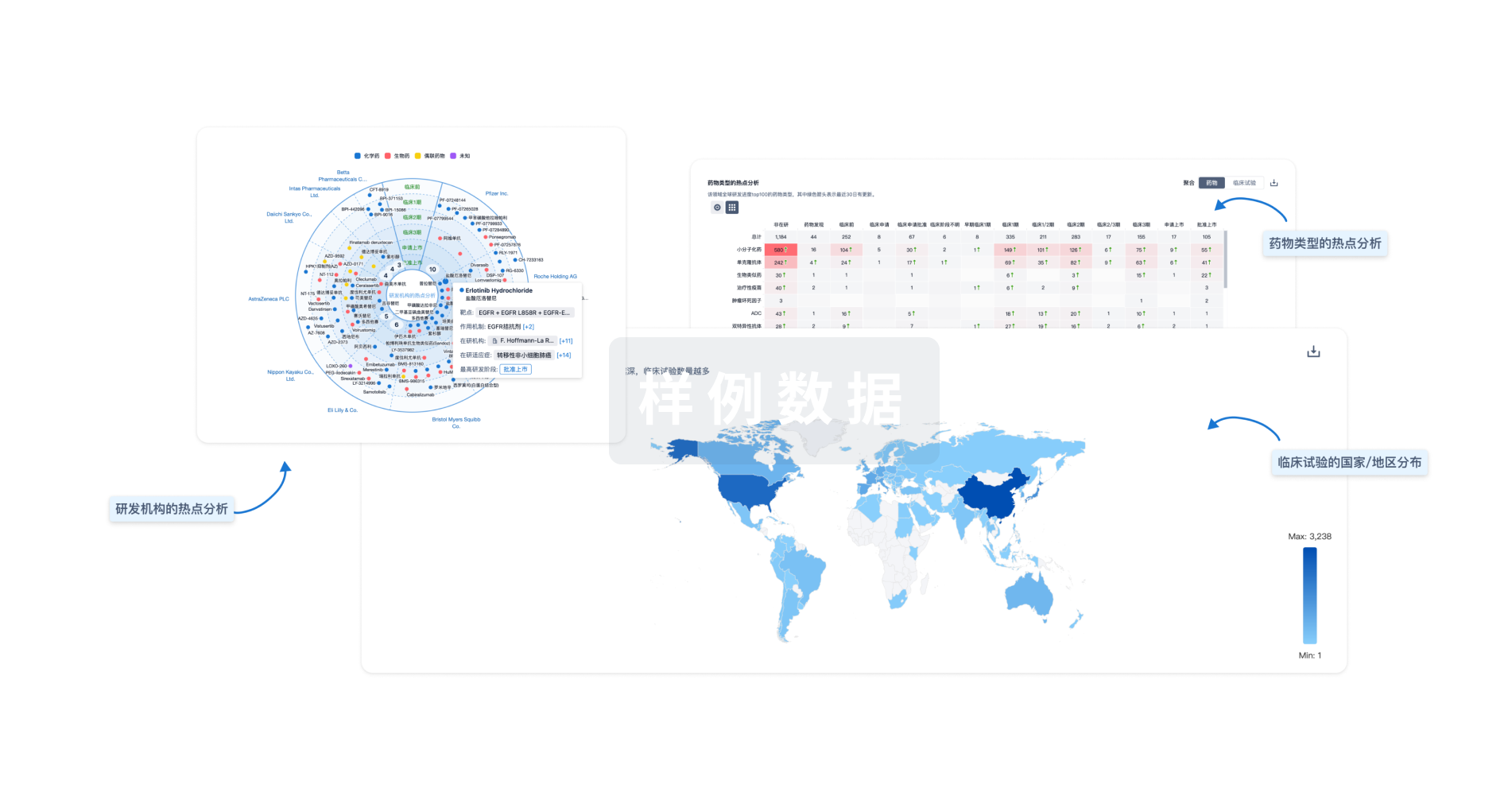
生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用





