预约演示
更新于:2025-09-09

Academy of Military Medical Sciences
更新于:2025-09-09
概览
标签
肿瘤
其他疾病
神经系统疾病
小分子化药
单克隆抗体
融合蛋白
疾病领域得分
一眼洞穿机构专注的疾病领域
暂无数据
技术平台
公司药物应用最多的技术
暂无数据
靶点
公司最常开发的靶点
暂无数据
| 排名前五的药物类型 | 数量 |
|---|---|
| 小分子化药 | 20 |
| 单克隆抗体 | 4 |
| mRNA | 2 |
| 放射与诊断药物 | 2 |
| 外泌体药物 | 2 |
关联
45
项与 中国人民解放军军事医学科学院 相关的药物作用机制 GABAA receptor激动剂 |
原研机构 |
最高研发阶段批准上市 |
首次获批国家/地区 日本 |
首次获批日期1988-03-29 |
作用机制 50S subunit抑制剂 |
在研机构 |
原研机构 |
在研适应症 |
非在研适应症- |
最高研发阶段批准上市 |
首次获批国家/地区- |
首次获批日期- |
靶点 |
作用机制 PDGFR激动剂 |
在研机构 |
原研机构 |
在研适应症 |
非在研适应症- |
最高研发阶段临床2期 |
首次获批国家/地区- |
首次获批日期- |
34
项与 中国人民解放军军事医学科学院 相关的临床试验CTR20220638
一项评价重组人血小板源生长因子凝胶(rhPDGF-BB)在糖尿病足溃疡患者中的初步有效性和安全性的、多中心、随机、双盲、安慰剂对照的Ⅱ期临床研究
评价试验药物在糖尿病足溃疡受试者中的初步有效性和安全性
开始日期2024-10-31 |
申办/合作机构  华芢生物科技(青岛)股份有限公司 华芢生物科技(青岛)股份有限公司 [+1] |
NCT07001969
Massoud-Goel Technique and Theory:
Percutaneous Trans-facet Fixation for Treatment of Degenerative Spine
Percutaneous Trans-facet Fixation Massoud-Goel Technique and Theory for Treatment of Degenerative Spine
开始日期2024-07-07 |
申办/合作机构 |
CTR20232035
注射用重组人神经生长因子在非动脉炎性前部缺血性视神经病变患者中的
多中心、随机、双盲、安慰剂合并基础治疗、平行对照 II 期临床试验
主要目的:在I期临床试验的基础上,在非动脉炎性前部缺血性视神经病变患者中进一步探讨其有效剂量,为III期临床试验安全性和有效性提供依据; 次要目的:评价注射用重组人神经生长因子在非动脉炎性前部缺血性视神经病变患者中的安全性。
开始日期2023-12-29 |
申办/合作机构  中国人民解放军军事医学科学院 中国人民解放军军事医学科学院 [+1] |
100 项与 中国人民解放军军事医学科学院 相关的临床结果
登录后查看更多信息
0 项与 中国人民解放军军事医学科学院 相关的专利(医药)
登录后查看更多信息
3,718
项与 中国人民解放军军事医学科学院 相关的文献(医药)2026-01-01·TALANTA
Michael-addition reaction triggering sensitive and facile detection of glutathione employing LSPR-photonic crystals
Article
作者: Peng, Yuan ; Zhu, Yanxi ; Gai, Xuejiao ; Xi, Dongmei ; Ju, Hong ; Han, Zhiyu ; Song, Yanqiu
Glutathione (GSH) plays a crucial role in various cellular functions, and its abnormal levels often indicate numerous diseases. Therefore, it's essential to develop a simple, rapid, and sensitive method to monitor GSH levels in serum for real-time tracking disease. In this work, a Michael-Addition reaction triggering GSH detection method was constructed based on a Localized Surface Plasmon Resonance enhanced opal Photonic Crystal (LSPR-PC). LSPR-PC was prepared through vertical deposition self-assembly method using the Gold nanoparticles (AuNPs) modified SiO2 microspheres, and the oxidized dopamine (o-DA), as the recognition element, was fixed on the LSPR-PC through Au-N bond, forming a LSPR-o-DA PC. The sulfhydryl group in GSH could undergo a Michael addition reaction with the oxidized dopamine on the PC surface, resulting in a reduction of the reflection peak intensity. The detection limit for GSH was 1.3 ng/mL. Real serum samples were detected using LSPR-o-DA PC, and a commercial GSH kit was chosen as a comparison. The detection results obtained through LSPR-o-DA PC method was consistent with that obtained through GSH kit, indicating that LSPR-o-DA PC method having a good accuracy. Notably, the entire assay process is completed within 20 min. The as-proposed LSPR-o-DA PC method is simple-operation, label-free, rapid and cost-effective, making it a promising tool for clinical diagnostics.
2025-12-31·Virulence
Multi-omics profiling of acute
Pseudomonas aeruginosa
pneumonia unmasks conventional NK cell depletion and stage-specific therapeutic targets
Article
作者: Wang, Yifeng ; Hu, Lingfei ; Zhou, Dongsheng ; Xiao, Nan ; Yang, Huiying ; Zong, Fuliang ; Su, Duo
Pseudomonas aeruginosa (PA) is a key pathogen in hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP), challenging clinical medicine. This study aims to elucidate the characteristics of the host's innate immune response following inhalational PA infection. We developed a mouse model by aerosolized intratracheal inoculation with PA and conducted a comprehensive analysis at the protein, cellular, and gene expression levels. Protein analysis revealed a substantial increase in inflammatory proteins in the bronchoalveolar lavage fluid and serum, indicating a robust inflammatory response in the lungs and systemic circulation. Cellular investigations showed an increase in neutrophils, monocytes, and alveolar macrophages during infection, whereas NK cells showed a marked reduction from 5.88% pre-infection to 2.41% at 24 h (p = 0.0102) and 1.55% by 48 h (p = 0.0023). To assess gene expression changes, RNA-sequencing technology was employed to map the temporal shifts in the transcriptional profile of the host lung post-infection. We analysed the expression patterns and dynamic transcriptional characteristics of differentially expressed genes (DEGs), describing the inflammation progression. Importantly, Through the analysis of single-cell RNA sequencing (scRNA-seq) datasets in public repositories, we observed the reduction in conventional natural killer (cNK) cells, rather than tissue-resident natural killer (trNK) cells in the early stages of PA infection. Sequential scRNA-seq analysis resolved NK-subset heterogeneity, revealing that cNK dominance (77.8% of total NK cells) under homeostasis collapsed to 9.2% within 24 h post-infection. Our findings establish cNK attrition as the earliest immune checkpoint failure in PA pneumonia and provide proof-of-concept for cNK-targeted immunotherapies to counteract lethal pulmonary infections. Keywords: Pseudomonas aeruginosa, aerosolized intratracheal inoculation, conventional NK cells, innate immunity, RNA-sequencing.
2025-12-31·Virulence
Plasmids of two novel incompatibility groups IncFII
pPROV114-NR
and Inc
pCHS4.1-3
from
Providencia
Article
作者: Chen, Fangzhou ; Zhou, Dongsheng ; Wang, Peng ; Zheng, Yali ; He, Jiaqi ; Yin, Zhe ; Yang, Fan ; Luo, Jing ; Lu, Xiuhui ; Gao, Bo ; Ma, Kejiao
This study presents the genetic structure of two incompatibility (Inc) groups found in Providencia: the newly discovered IncFIIpPROV114-NR and the newly designated IncpCHS4.1-3. An extensive genomic comparison was performed on all 14 plasmids (three IncFIIpPROV114-NR plasmids and 11 IncpCHS4.1-3 plasmids) from Providencia, including 12 newly sequenced in this study and two from GenBank. Three IncFIIpPROV114-NR plasmids had similar conserved backbones but differed in accessory modules. The 11 IncpCHS4.1-3 plasmids fell into two groups according to differences in the conserved genes of the plasmid backbone. The accessory modules of 11 IncpCHS4.1-3 plasmids showed significant diversity, indicating numerous gene gains and losses, including in the Tn1696-related region, in Tn7504, in a 17.3-kb sul2 region, and a 63.6-kb blaNDM-1 region. A minimum of 45 obtained antimicrobial resistance genes (ARGs) were identified in 13 of the 14 plasmids, covering resistance to 14 classes of antimicrobials and heavy metals. Five new mobile genetic elements (MGEs) were identified, including In2168, In1790, Tn7500, Tn7501, and Tn7502. Additionally, three MGEs, Tn7499, Tn7503, and Tn7504 were newly designated. These two Inc group plasmids integrate abundant accessory modules that allow them to accumulate and distribute ARGs and improve the survivability of Providencia under the pressure of drug selection.
34
项与 中国人民解放军军事医学科学院 相关的新闻(医药)2025-07-18
GA, UNITED STATES, July 18, 2025 /
EINPresswire.com
/ -- A deadly tick-borne illness, severe fever with thrombocytopenia syndrome (
SFTS
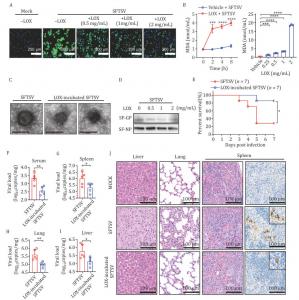
), continues to claim lives with no specific antiviral treatment. However, a breakthrough study reveals that lipoxygenase (LOX), an enzyme previously known for its role in inflammation, etc., can effectively halt the progression of SFTS virus (SFTSV) infection. By disrupting the viral envelope through lipid peroxidation, LOX prevents the virus from infecting host cells, offering a potential new strategy for combating this deadly disease. This discovery could have far-reaching implications for the treatment of other dangerous enveloped viral infections as well.
Severe fever with thrombocytopenia syndrome (SFTS) was reported in Asia, with a fatality rate that can reach as high as 50%. Despite efforts with existing antiviral drugs, no treatment has consistently proven effective. Lipoxygenase (LOX), a natural enzyme involved in the oxidation of fatty acids, has recently attracted attention for its antiviral properties. Research has shown that LOX can alter the structure of viral envelopes via lipid peroxidation, preventing the virus from entering host cells. Given the urgent need for effective therapies, this discovery suggests that LOX might be a key player in the battle against SFTSV and similar viral infections. Based on these challenges, further research is essential to understand how LOX can be leveraged in antiviral treatment.
Published (DOI:
10.1093/procel/pwae061
) in Protein & Cell (October 2024), a letter-style study reveals the remarkable antiviral potential of lipoxygenase (LOX) in fighting the severe fever with thrombocytopenia syndrome virus (SFTSV). The new research, led by teams from the Academy of Military Medical Science and other esteemed institutions, shows that LOX can disrupt the viral lipid envelope through peroxidation, preventing the virus from infecting host cells. These findings could lead to the development of innovative antiviral therapies, not only for SFTSV but also for other enveloped viruses.
The study demonstrated LOX's powerful ability to prevent SFTSV infection in lab-grown cells. By catalyzing lipid peroxidation, LOX disrupts the viral envelope, which is essential for the virus to bind to and infect host cells. The researchers tested this mechanism in both in vitro experiments and a mouse model, where they observed a significant reduction in viral replication and less severe tissue damage in mice treated with LOX-pre-incubated virus. LOX pre-treatment also showed promise in combating other enveloped viruses, including influenza and vesicular stomatitis virus, highlighting its broad-spectrum antiviral properties. These results suggest that LOX could be a powerful tool for stopping viral infections at multiple stages of the disease.
Dr. Wei Liu, one of the corresponding authors of the study, explains: This research reveals a new potential weapon against viral infections. LOX is capable of destabilizing the viral envelope by catalyzing lipid peroxidation, rendering the virus unable to infect host cells. This discovery is exciting because it not only opens new possibilities for treating SFTSV but also paves the way for future antiviral therapies targeting other dangerous enveloped viruses.
The findings from this study position LOX as a promising candidate for future antiviral therapies. Its ability to disrupt the viral membrane offers a novel approach to treating SFTSV, which has proven difficult to manage with existing drugs. Furthermore, LOX's broad-spectrum activity against other enveloped viruses suggests that it could play a significant role in tackling various viral infections. Future research will be crucial to refine LOX-based treatments, optimize its use in clinical settings, and explore its full potential as a therapeutic tool for viral diseases.
DOI
10.1093/procel/pwae061
Original Source URL
https://doi-org.libproxy1.nus.edu.sg/10.1093/procel/pwae061
Funding information
The study was supported by the National Natural Science Foundation of China (22121003).
Lucy Wang
BioDesign Research
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability
for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this
article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
临床研究
2025-07-17
·云南白药
近期,中药龙头云南白药(000538)发布公告称:云南白药集团股份有限公司控股子公司云白药征武科技(上海)有限公司于近日收到国家药品监督管理局下发的《药物临床试验批准通知书》(通知书编号:2025LP01506、2025LP01507)。经审查,征武科技的JZ-14胶囊(以下简称“本品”)临床试验申请符合药品注册的有关要求,同意本品开展溃疡性结肠炎的临床试验。标志着云南白药在创新药领域的研发再进一步。据了解,JZ-14胶囊为征武科技自主研发的化学1类创新药,是First-in-Class(首创新药)的小分子免疫调节剂。JZ-14可选择性结合特定的蛋白靶点,抑制其下游AKT/NF-κB等信号通路,从而在溃疡性结肠炎与多种肿瘤模型中展现出显著的免疫调节及抗增殖活性。云南白药作为国内知名的百年药企,近年来积极拥抱创新,推动业务高质量发展。在中医药振兴发展迎来天时、地利、人和的历史性机遇下,作为行业领军企业的云南白药,始终将创新研发作为公司高质量发展的基石。一方面,作为云南省中药材产业发展的“链主”企业,云南白药深入贯彻省委、省政府决策部署,通过科技赋能,充分挖掘云药资源优势,将资源禀赋转化为产业竞争优势,建设云南品牌中药材产业新生态;另一方面,坚持打好中药、创新药两张牌。深耕中药二次开发,大力发展传统中药和民族药,强化创新药物研发、AI辅助药物设计,不断拓宽创新力的内涵,书写着传统中药企业向创新型医药集团转型的生动实践。透过其创新布局,让市场看到了一家百年药企以创新为内核,担当中药现代化发展重任的坚定决心和强大实力。经典产品焕新升级 夯实发展根基在云南白药的产品矩阵中,云南白药气雾剂、云南白药胶囊、气血康口服液等共同构建起了坚固的基本盘。据公司2024年年报,公司药品事业群实现主营业务收入69.24亿元,同比增长11.8%。其中,前述核心和主要产品均在高基数基础上实现同比显著增长。而云南白药的短期创新成果,正依托于这些成熟产品。这些已上市品种的二次创新,不仅保证了云南白药的创新效率,也最大化释放其商业潜力。据披露,截至2024年底云南白药在研项目中,涉及二次开发的中药大品种共有11个,开展项目25个。以气血康项目为例,在临床研究中,发现气血康口服液在改善血管内皮功能、血液流变学指标等方面有良好效用,北京大学和云南白药联合启动了国内首个针对中药改善血管健康有效性和安全性的临床研究。通过对临床研究109例患者的数据收集结果显示,稳定型冠心病患者在连续服用气血康口服液4周后,在血管内皮功能方面,气血康口服液显著提高了稳定型冠心病患者的反应性充血指数(RHI),表明血管内皮功能有所改善。公司气血康改善血管健康有效性和安全性临床研究相关重要成果《Effectiveness and safety of Qixuekang Oral Liquid on vascular health》发表于专注转化医学领域的国际一流期刊《Journal of Translational Internal Medicine》(JTIM)。同时,云南白药与中国人民解放军军事科学院军事医学研究院开展 “气血康口服液急进高原习服能力临床研究”,就治疗高原病领域的临床应用、维持和增强高原高寒人员作业效能探索新路径。舒列安胶囊为云南白药集团独家品种,在北京大学第一医院开展了治疗慢性前列腺炎多中心、开放、自身对照临床试验,初步结果表明,舒列安胶囊具有良好的安全性,在使用4周后,治疗慢性前列腺炎,尤其是改善疼痛、尿频和尿不尽等症状方面效果显著,可有效改善患者的生活质量。云南白药(散剂)、胶囊在糖尿病足、骨骼疼痛等方面的临床研究,宫血宁胶囊在治疗异常子宫出血、减少药物流产后阴道流血等方面的临床研究均取得重要进展。近期,云南白药与深圳大学总医院合作的“云南白药气雾剂治疗闭合性肋骨骨折疼痛的随机、双盲、安慰剂对照、多中心临床试验”作为云南省科技厅重大科技专项计划项目在中国工程院姜保国院士的带领下已顺利开展;另一项由姜保国院士牵头,深圳大学总医院作为组长单位,联合北京大学人民医院、上海市第六人民医院、四川大学华西医院、宁波市第六人民医院等11家参研单位的“云南白药胶囊治疗踝关节周围骨折肢体肿胀临床多中心试验”项目进入了临床实施阶段。值得注意的是,对已上市品种进行二次开发,不是简单的产品改良,而是通过科技创新重新定义产品的临床价值和市场价值。当前,中药行业面临同质化竞争困局、质量标准升级压力、市场环境巨变、政策机遇窗口及培育国际竞争力等特殊外部因素。在这一背景下,中药二次开发不是选择题,而是必答题。通过二次开发拓展或细化产品在某疾病领域的临床应用,其意义不仅在于单个产品的价值提升,更在于推动整个产业从经验型向科技型转变,从数量型向质量型跨越。这既是产业生存的需要,更是时代发展的必然。这种对传统产品的持续创新,不仅是对百年品牌的守护,更是对“守正创新”这一中医药发展原则的最好践行。创新突破 强化中药企业科技创新主体地位如果说短期创新是夯实基础,那么中长期的创新布局则展现了云南白药引领行业发展的实力与担当。以持续打造云南白药透皮制剂明星产品为核心,云南白药围绕全三七片、附杞固本膏、贴膏剂、新型创面止血材料与动脉止血凝胶打造了系列中期创新项目。去年,公司完成全三七片II期临床试验入组362例,同步完成药学相关研究;附杞固本膏项目已进入Ⅲ期临床试验。贴膏剂项目共有4个在研品种,累计获得专利4项。其中氟比洛芬凝胶贴膏获得临床实验批件并完成临床预试验,洛索洛芬钠凝胶贴膏完成中试研究。新型创面止血材料与动脉止血凝胶项目完成1款急救止血绷产品带完成注册申报,获得二类医疗器械注册证。今年3月,国务院办公厅印发《关于提升中药质量促进中医药产业高质量发展的意见》。国家中医药局在政策解读文章中特别提到,要强化中药企业科技创新主体地位,发挥龙头企业示范引领作用,促进科技成果转化。同时,要结合中医优势病种,聚焦重大慢病、重大疑难疾病、新发突发传染病、特殊环境疾病等,推出一批临床疗效突出、竞争优势显著的中药创新药。在此背景下,云南白药的中期创新项目既体现了龙头企业对创新责任的积极担当,又牢牢抓住了中药现代化的发展机遇。其创新路径不仅为企业未来发展蓄能,更通过示范效应带动整个产业向高质量发展迈进。随着这些项目陆续进入收获期,云南白药有望在中药创新领域树立新的标杆,为中国中医药事业贡献更多"白药智慧"。全链路创新布局保障企业未来高质量发展从长远来看,云南白药把握市场先机精准布局创新药。在核药赛道,用于PET/CT显像诊断可疑前列腺癌患者的INR101诊断核药项目2024年5月获得临床批件,11月完成I/IIa期临床总结报告,结果显示产品具备优异的稳定性和安全性,目前已进入III期临床。用于治疗转移性去势抵抗前列腺癌患者的INR102治疗核药项目完成符合IND申请要求的非临床研究及报告,于2024年11月递交pre-IND沟通交流申请,并2025年4月获得药物临床试验批准,已进入临床。另外,云南白药引进了抗体药物KA-1641的相关专利,并将在全球范围内对标的产品进行研究开发、生产和商业化活动。这些项目的推进,将进一步丰富云南白药产品线,提升市场竞争力。面向更长远的发展,创新是云南白药实现战略目标的核心驱动力,经历百年积淀云南白药已经进入新的发展周期,2024年重磅发布《2024 - 2028战略规划》,明确提出通过 “2 + 3” 战略两步走策略,实现营收、利润、资产规模等指标增长,推动企业向国内领先、世界一流的现代医药产业集团迈进。近年来公司高度重视研发投入,云南白药中央研究院下设昆明、北京、上海和无锡四大研发中心,通过相互联动,不断提高自主创新能力,逐步构建具有世界影响力的现代化研发体系。昆明研发中心充分利用本地得天独厚的动植物资源,聚焦中药、天然药物的研究工作,保持公司在天然植物提取方面的传统研发优势,持续提升云南白药自主研发能力;北京以北京大学-云南白药国际医学研究中心为平台,连通创新产学研链条,紧盯世界前沿技术研究,探索校企合作新机制,加速学术研究成果转化,吸引国际一流的科学家加入,加速科研成果转化,打造一个富有竞争力的医学品牌;上海中心以创新药物中心、云白药征武科技(上海)有限公司为科研平台,重点研究领域为创新药物,建设公司的产品开发和商务拓展能力,布局创新孵化体系,培育新兴业务板块;无锡中心则主要深耕切合市场需求、技术领先、专业验证的医疗器械产品。在技术应用上,云南白药紧跟时代步伐,积极探索AI 技术在药物研发中的应用。例如,与华为云合作打造中医药行业雷公大模型,利用其强大的数据处理与分析能力,助力中药新药研发过程中的靶点发现、药物分子设计等关键环节,大幅缩短研发周期,提高研发成功率,推动中药研发从经验驱动向数据与科技驱动转变,为公司源源不断地注入创新活力。聚焦到口腔健康领域,“北大口腔医学-云南白药口腔健康联合实验室”近日在北京大学口腔医院成立。通过引入前沿的基础研究理论,实验室将深度赋能云南白药在口腔垂直领域构建新的基础研究能力,共同推动口腔健康领域的技术突破与创新转化,助力口腔健康产业的提升。站在新的历史起点上,从"百年秘方"到"创新药企",云南白药的转型之路还在继续。但可以确定的是,在这条以创新为底色的发展道路上,这家企业正以其坚定的创新定力、雄厚的创新实力和巨大的创新潜力,担当起引领中医药产业高质量发展的时代重任。转发自:每日经济新闻往期阅读推荐·省医药“云找药”与云南红塔银行APP跨界联动 健康生活 一“触”即达·传承红色基因 致敬光荣岁月——云南白药集团党委向“光荣在党50年”老党员颁发纪念章·产学研共探中药材高质量发展破局之路 云南省“云鉴本草”中药材鉴创活动第二期举行·第十三届消化外科学术会议在昆圆满落幕 云南白药助力共绘精准外科新图景·云南白药入选联合国(UN)全球人工智能向善峰会"AlforGood"案例·云南白药集团携手丽江玉龙县共筑中药资源发展新高地
财报申请上市
2025-06-12
·药时空
2025年6月12日,君实生物发布公告称其控股子公司上海君拓生物收到国家药品监督管理局核准签发的《受理通知书》,JT118注射液(项目代号“JT118”)的临床试验申请获得受理,受理号为CXSL250047。据公告显示,JT118是由猴痘病毒抗原A35(属于胞外囊膜病毒抗原)和M1(胞内成熟病毒抗原)串联融合组成的“二合一”重组蛋白疫苗,拟主要用于预防猴痘病毒感染。2023年10月,君实生物与北京大学、中国科学院微生物研究所、山西高等创新研究院、北京航空航天大学达成合作,共同开发猴痘重组蛋白疫苗。猴痘是一种由猴痘病毒感染所致的人兽共患病毒性疾病,猴痘病毒归类于痘病毒科正痘病毒属,痘科病毒复制过程中,产生两种形态不同的病毒粒子:胞外囊膜病毒和胞内成熟病毒,且都有感染性。猴痘病毒分为分支Ⅰ型和分支Ⅱ型。传染源包括猴痘患者及感染的啮齿类动物、猴和猿等灵长类动物。床表现主要有发热、皮疹、淋巴结肿大等症状。据悉,JT118既保留了抗原A35和M1两种天然抗原的表位结构,又通过多抗原融合大幅增加了分子量,更有利于免疫激发。临床前研究结果表明,JT118在大小动物上接种均可以产生高水平的结合抗体、痘苗病毒/猴痘病毒中和抗体和细胞免疫激活,对痘苗病毒/猴痘病毒感染的大小动物模型均具有显著的保护作用,且安全性良好。既往猴痘疫情主要在非洲地区流行,2022年后逐渐扩散至全球大多数国家和地区。2022年7月,世界卫生组织(WHO)宣布猴痘疫情构成“国际关注的突发公共卫生事件”;2024年8月,WHO再次宣布猴痘疫情构成“国际关注的突发公共卫生事件”。当时时间6月5日,WHO总干事谭德塞在国际卫生条例紧急委员会会议上表示,自2024年初以来,猴痘疫情在多个国家持续扩散,全球已报告逾3.7万例确诊病例,并造成125人死亡。疫苗接种是疫情防控的重要措施之一,目前,全球至少已有4款猴痘疫苗获批用于预防猴痘感染,分别为OrthopoxVac、MVA-BN、LC16m8、ACAM2000,这4款疫苗原本均是用于预防天花而被研发的。此外,还有多种减毒活疫苗、mRNA疫苗、DNA疫苗和重组亚单位疫苗等处于研发中。据不完全统计,全球正在进行的猴痘相关临床试验超过30余项。至于国内,目前尚无预防猴痘病毒感染疫苗获批上市。不过,有多家生物药企和科研机构已投身于猴痘疫苗研发,此前的主要研究方向包括复制缺陷性猴痘减毒活疫苗和猴痘mRNA疫苗,而君实的猴痘重组蛋白疫苗有望成为该技术路线国内首款进入临床的疫苗。2024年9月,国药集团中国生物上海生物制品研究所自主研发的MVA株猴痘减毒活疫苗获得国家药品监督管理局签发的临床试验通知书,这是我国首款获批临床的猴痘疫苗。目前,该疫苗正处于Ⅰ期临床(招募中),目标入组人数120人,已入组人数69人。同年12月,由国药集团中国生物北京生物制品研究所与中国疾病预防控制中心病毒病预防控制所合作研发的复制缺陷型猴痘疫苗获批临床试验。此外,据公开消息称,国药集团宣布研发了3种针对猴痘的候选mRNA疫苗。中山大学附属第七医院研发团队正开发多价猴痘mRNA疫苗。智飞生物表示,公司在研管线内的猴痘疫苗产品处于临床前研究阶段。康希诺生物表示,公司已完成猴痘疫苗抗原初步筛选等前期工作,具备快速开发及制备疫苗的潜力及能力。在专利布局方面,国内企业康希诺、易慧生物、鹏泊生物,国内高校中国人民解放军军事科学院军事医学研究院、中国科学技术大学、中国科学院上海免疫与感染研究所等,均布局有猴痘病毒mRNA疫苗,包括多价、多抗原的猴痘RNA疫苗,快速高效增强免疫应答的猴痘mRNA免疫原组合物等。参考资料:[1]君实生物公司公告等.[2] 猴痘防控科普知识(2024年版). 国家疾控局. 2024年09月14日.[3] 猴痘太可怕了!首个国产“猴痘疫苗”获准临床,中国有三款. 药物杂评. 2024年09月11日.[4] Zhai YQ, Han YZ, Wang WL, Tan WJ. Advancements in Mpox Vaccine Development: A Comprehensive Review of Global Progress and Recent Data. Biomed Environ Sci. 2025 Feb 20;38(2):248-254. doi: 10.3967/bes2024.121. PMID: 40159176.[5] Shafaati M, Forghani S, Shahsavand Davoudi A, Samiee R, Mohammadi K, Akbarpour S, Seifi A, Salehi M, Zare M. Current advances and challenges in mpox vaccine development: a global landscape. Ther Adv Vaccines Immunother. 2025 Jan 27;13:25151355251314339. doi: 10.1177/25151355251314339. PMID: 39872308; PMCID: PMC11770767.[6] 猴痘病毒mRNA疫苗开发进展!附完整中国专利清单(截至2025.4.13). 核药联盟. 2025年04月14日.[7] 舆情看点|病毒毒株变异 疫情警报拉响 猴痘相关疫苗、治疗药物、检测试剂研发受关注. 中国医药报. 2024年09月23日.[8] 世卫组织警告猴痘疫情持续蔓延,呼吁全球筹集1.47亿美元加强应对.界面新闻.2025年06月06日.识别微信二维码,可添加药时空小编请注明:姓名+研究方向!
疫苗信使RNA上市批准临床研究
100 项与 中国人民解放军军事医学科学院 相关的药物交易
登录后查看更多信息
100 项与 中国人民解放军军事医学科学院 相关的转化医学
登录后查看更多信息
组织架构
使用我们的机构树数据加速您的研究。
登录
或

管线布局
2025年10月02日管线快照
管线布局中药物为当前组织机构及其子机构作为药物机构进行统计,早期临床1期并入临床1期,临床1/2期并入临床2期,临床2/3期并入临床3期
药物发现
33
5
临床前
临床1期
3
3
临床2期
批准上市
1
32
其他
登录后查看更多信息
当前项目
| 药物(靶点) | 适应症 | 全球最高研发状态 |
|---|---|---|
复方萘酚阿奇片 ( 50S subunit ) | 疟疾 更多 | 批准上市 |
重组人神经生长因子 (四川自豪时代药业) ( p75NTR ) | 视神经损伤 更多 | 临床2期 |
重组人血小板源生长因子(北京华芢生物) ( PDGFRs ) | 糖尿病足溃疡 更多 | 临床2期 |
咪达唑仑 ( GABAA receptor ) | 镇静 更多 | 临床1期 |
知母皂苷BII | 血管性痴呆 更多 | 临床1期 |
登录后查看更多信息
药物交易
使用我们的药物交易数据加速您的研究。
登录
或

转化医学
使用我们的转化医学数据加速您的研究。
登录
或

营收
使用 Synapse 探索超过 36 万个组织的财务状况。
登录
或

科研基金(NIH)
访问超过 200 万项资助和基金信息,以提升您的研究之旅。
登录
或

投资
深入了解从初创企业到成熟企业的最新公司投资动态。
登录
或

融资
发掘融资趋势以验证和推进您的投资机会。
登录
或

Eureka LS:
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用


