预约演示
更新于:2025-01-23
Immune Cell Therapy, Inc.
私营公司|
2003|
Illinois, United States
私营公司|
2003|
Illinois, United States
更新于:2025-01-23
概览
标签
肿瘤
免疫系统疾病
血液及淋巴系统疾病
自体CAR-T
CAR-T
疾病领域得分
一眼洞穿机构专注的疾病领域
暂无数据
技术平台
公司药物应用最多的技术
暂无数据
靶点
公司最常开发的靶点
暂无数据
| 排名前五的药物类型 | 数量 |
|---|---|
| 自体CAR-T | 1 |
| CAR-T | 1 |
| 排名前五的靶点 | 数量 |
|---|---|
| CD30(肿瘤坏死因子受体超家族成员8) | 1 |
| CD19(B淋巴细胞抗原CD19) | 1 |
关联
6
项与 Immune Cell Therapy, Inc. 相关的药物靶点 |
作用机制 CD30抑制剂 |
非在研适应症- |
最高研发阶段临床1期 |
首次获批国家/地区- |
首次获批日期- |
靶点 |
作用机制 CD19抑制剂 |
非在研适应症- |
最高研发阶段临床1期 |
首次获批国家/地区- |
首次获批日期- |
靶点- |
作用机制 细胞替代物 |
在研机构- |
在研适应症- |
非在研适应症 |
最高研发阶段终止 |
首次获批国家/地区- |
首次获批日期- |
4
项与 Immune Cell Therapy, Inc. 相关的临床试验NCT03383965
A Clinical Study of CD30 Targeted CAR-T in Treating CD30-Expressing Lymphomas
CAR-T cells have been validated effective in treating CD19 positive B cell lymphoma. Other lymphomas like Hodgkin's lymphoma and anaplastic large cell lymphoma are CD30 positive. In this study, a newly CD30 targeted CART therapy ICAR30 is designed to specifically kill those CD30 expressing malignancies including Hodgkin's lymphoma and CD30+ anaplastic large cell lymphoma. The subjects will receive several doses of autologous ICAR30 T cells infusion and then the safety, treating effects and lasting period of these cells in vivo will be evaluated.
开始日期2017-03-01 |
申办/合作机构 |
NCT03383952
A Phase I Trial of CD19 Targeted ICAR19 T Cells in Patients With CD19+ Leukemia and Lymphoma.
Immunotherapy offers an extremely precise approach with the potential to eliminate cancer cells specifically. The newly designed CD19 targeted ICAR19 T cells can specifically kill CD19+ tumor cells. ICAR19 CART used the second generation of CART designation. In this study, the participants will receive several doses of autologous ICAR19 T cells and the investigators will determine the safety and therapeutic effects of these cells.
开始日期2017-03-01 |
申办/合作机构 |
NCT02211027
Active Immunization of Patients With Head and Neck Squamous Cell Carcinoma (HNSCC) Using Fibroblasts Transfected With DNA From Autologous Tumor
Hypothesis The incidence of toxicity in patients receiving the tumor DNA-transfected fibroblast vaccine will be acceptably low and the immunologic response rate sufficiently high to warrant further study of this therapy
The study of the vaccine will proceed in two stages after the method of Simon (102). In the first stage, 15 patients will be accrued and treated. If two or fewer objective immunologic responses occur, the study will be terminated. If 3 or more responses are observed, the study will proceed to the second stage, accruing an additional 22 patients. If the second stage is complete and a total of 9 or more immunologic responses are observed among the 37 patients treated, the treatment response rate for the vaccine will be considered high enough to warrant further study. Conversely, if the evaluation of the vaccine concludes at the first stage, or if 8 or fewer total immunologic responses occur after completing the second stage, the vaccine will not be considered for further study.
The study of the vaccine will proceed in two stages after the method of Simon (102). In the first stage, 15 patients will be accrued and treated. If two or fewer objective immunologic responses occur, the study will be terminated. If 3 or more responses are observed, the study will proceed to the second stage, accruing an additional 22 patients. If the second stage is complete and a total of 9 or more immunologic responses are observed among the 37 patients treated, the treatment response rate for the vaccine will be considered high enough to warrant further study. Conversely, if the evaluation of the vaccine concludes at the first stage, or if 8 or fewer total immunologic responses occur after completing the second stage, the vaccine will not be considered for further study.
开始日期2016-09-01 |
申办/合作机构 |
100 项与 Immune Cell Therapy, Inc. 相关的临床结果
登录后查看更多信息
0 项与 Immune Cell Therapy, Inc. 相关的专利(医药)
登录后查看更多信息
1
项与 Immune Cell Therapy, Inc. 相关的文献(医药)2012-05-20·Journal of Clinical Oncology
New clinical trial of DNA-based vaccines for patients with non-small cell lung cancer.
作者: Cohen, Edward P. ; Cohen, Jonathan M.
100 项与 Immune Cell Therapy, Inc. 相关的药物交易
登录后查看更多信息
100 项与 Immune Cell Therapy, Inc. 相关的转化医学
登录后查看更多信息
组织架构
使用我们的机构树数据加速您的研究。
登录
或
管线布局
2025年02月01日管线快照
管线布局中药物为当前组织机构及其子机构作为药物机构进行统计,早期临床1期并入临床1期,临床1/2期并入临床2期,临床2/3期并入临床3期
临床1期
2
4
其他
登录后查看更多信息
当前项目
登录后查看更多信息
药物交易
使用我们的药物交易数据加速您的研究。
登录
或
转化医学
使用我们的转化医学数据加速您的研究。
登录
或
营收
使用 Synapse 探索超过 36 万个组织的财务状况。
登录
或
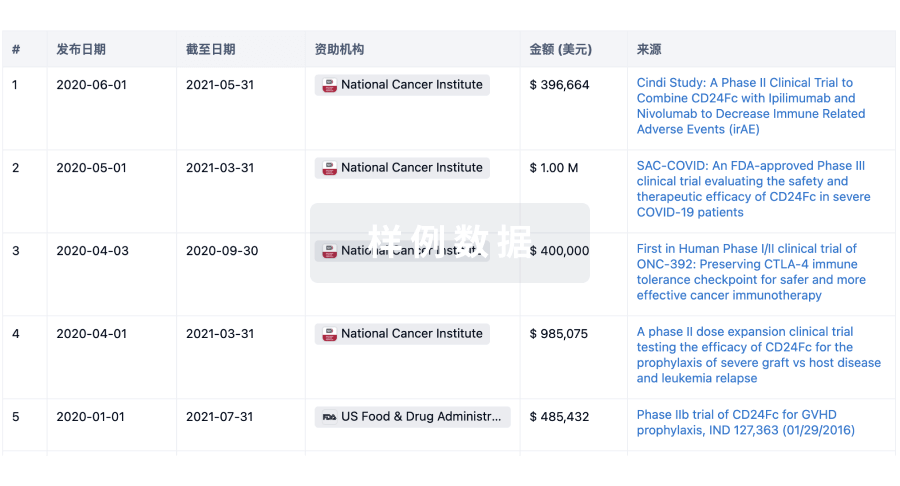
科研基金(NIH)
访问超过 200 万项资助和基金信息,以提升您的研究之旅。
登录
或
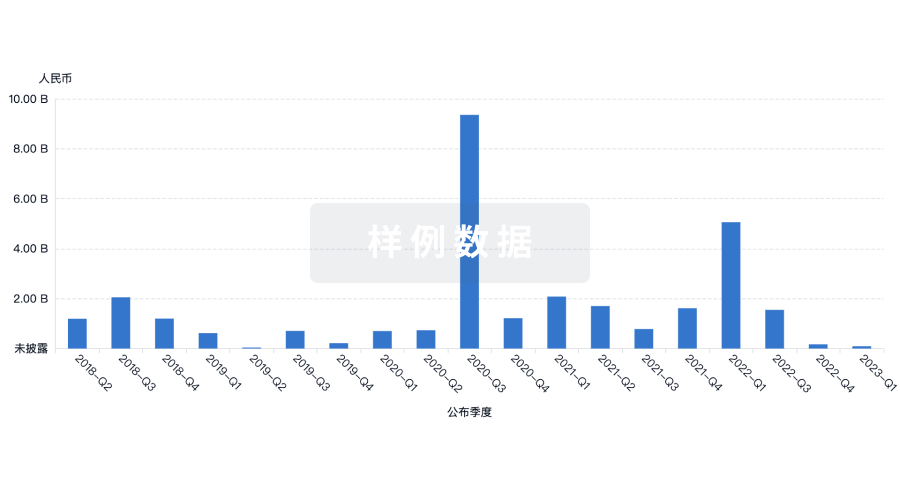
投资
深入了解从初创企业到成熟企业的最新公司投资动态。
登录
或
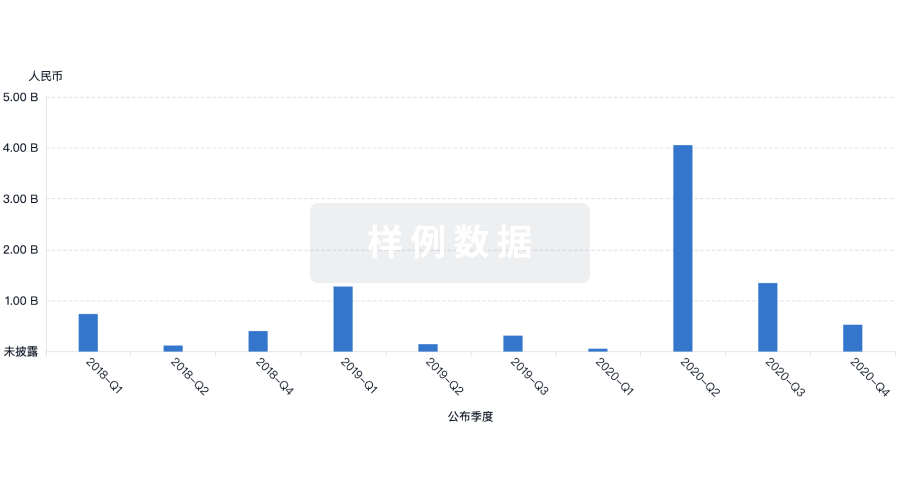
融资
发掘融资趋势以验证和推进您的投资机会。
登录
或
来和芽仔聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用
