UPDATED: Eicos Sciences' Aurlumyn scores first FDA approval to treat severe frostbite
2024-02-14
上市批准临床结果
Preview
来源: FiercePharma
Eicos Sciences of California has gained the first approval for severe frostbite with Aurlumyn, a drug that was originally approved in 2004 for pulmonary arterial hypertension.
Over the last month, for those watching HBO’s macabre “True Detective: Night Country”—a TV series set in a harsh, sunless winter in a fictional Alaskan town—a continual theme is frostbite.
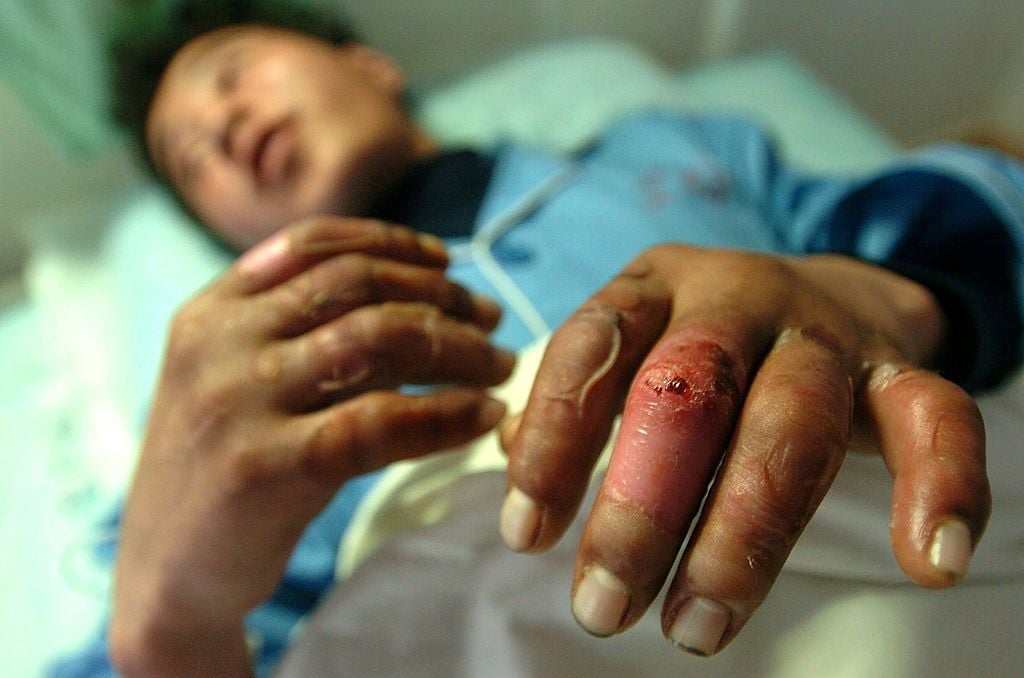
So the timing is right for the FDA to approve the first medicine for severe frostbite, which is caused by exposure to extreme cold. The U.S. regulator has signed off on Eicos Sciences' Aurlumyn (iloprost), an injected treatment to combat severe cases of frostbite in adults and reduce their risk of amputation of the fingers or toes.
As a vasodilator, iloprost opens blood vessels and prevents blood from clotting. In 2004, the drug, which was originally developed by California company CoTherix, won an approval to treat pulmonary arterial hypertension (PAH). Known commercially as Ventavis, it was then the only inhaled therapy for PAH in the U.S.
Eicos, which is a subsidiary of California-based CiVi Biopharma, has reformulated iloprost into an injected treatment, developing it for frostbite since the inception of the company in 2017.
Maryland-based Eicos also is investigating iloprost as a treatment for a circulation disorder, extracorporeal membrane oxygenation (ECMO), and for systemic sclerosis, another disease that can leave patients at risk of amputation of their fingers or toes.
Aurlumyn’s endorsement is based on a trial of 47 adults with severe frostbite who were split into three groups. One received iloprost intravenously for six hours daily for up to eight days. The two other groups received other medications that are unapproved for frostbite, with one of the groups also receiving iloprost and the other not.
Severe frostbite removes all sensation of cold or discomfort and can cause muscles and joints to stop working. It can affect all layers of the skin, with blisters forming 24 hours after exposure and tissues turning black as they die, according to the Mayo Clinic.
Editor's note: The original version of this story, based on a release from the FDA, credited Johnson & Johnson as the owner of Aurlumyn (iloprost).
更多内容,请访问原始网站
文中所述内容并不反映新药情报库及其所属公司任何意见及观点,如有版权侵扰或错误之处,请及时联系我们,我们会在24小时内配合处理。
靶点
-药物
热门报告
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。