Palisade's ulcerative colitis drug prevents symptoms in mice, is nontoxic in dogs
2024-05-23
临床申请临床研究
Preview
来源: FierceBiotech
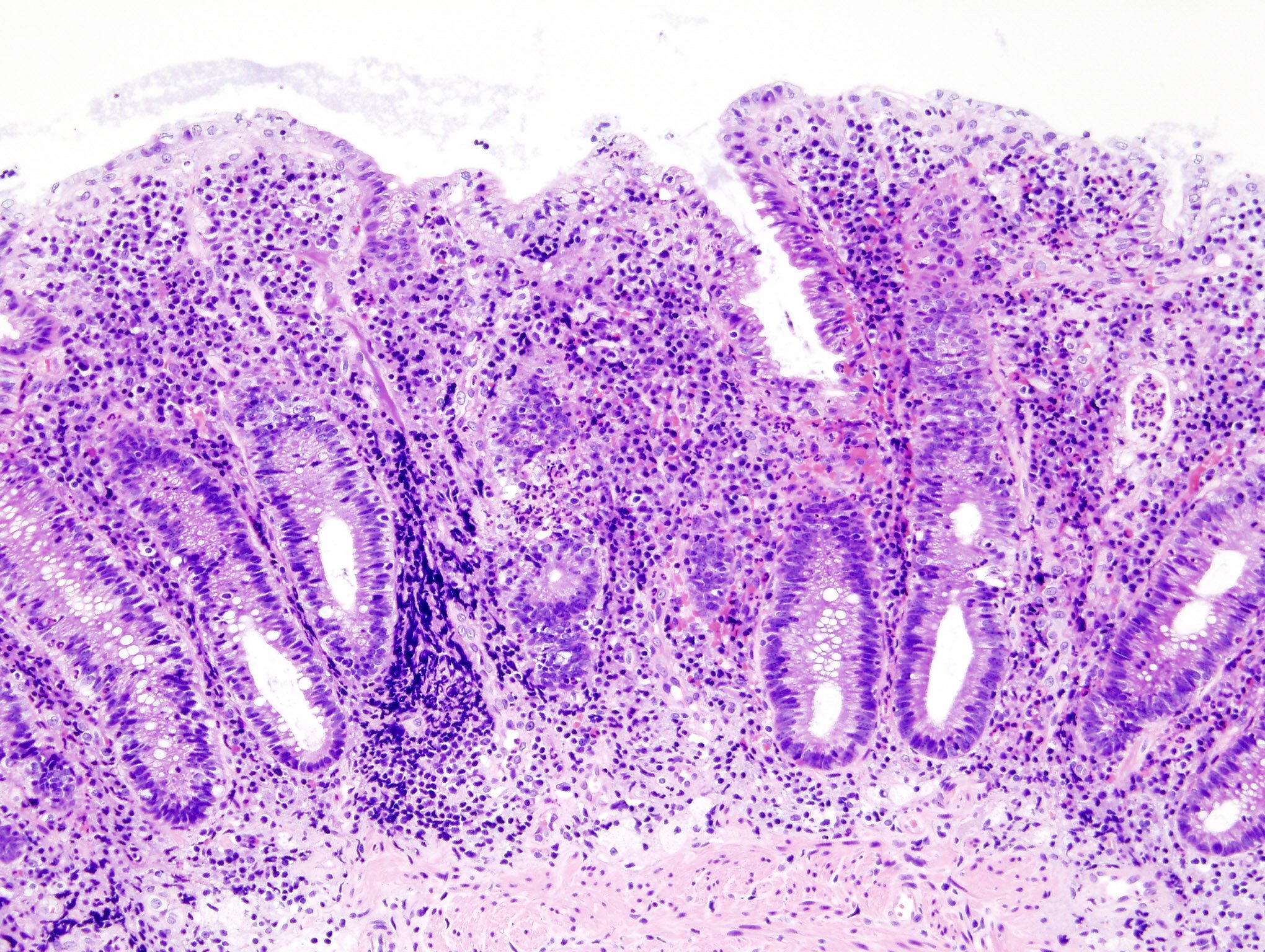
Besides results from mouse studies, Palisade Bio showed PALI-2108 was nontoxic in dogs and that it has a larger therapeutic window than the compound it metabolizes to, PALI-0008.
Palisade Bio’s PDE4 inhibitorPDE4 inhibitor to treat the inflammatory bowel disease ulcerative colitis appears to be effective in mice, according to new data.
The drug engaged its target as well as another PDE4 inhibitorPDE4 inhibitor, apremilast—commercialized by Amgen as Otezla—a psoriasis drug that has also been previously tested as an ulcerative colitis treatment.
Palisade presented the mouse studies as a poster (PDF) during the annual Digestive Disease Week conference in Washington, D.C., along with data showing that PALI-2108 was nontoxic in dogs and has a larger therapeutic window than the compound it metabolizes to, PALI-0008.
“The findings from this preclinical study add to our growing body of encouraging data for PALI-2108 and further bolster our confidence in its potential in the treatment of [ulcerative colitis],” Mitch Jones, M.D., Ph.D., chief medical officer at Palisade, said in the press release.
PALI-2108 is Palisade’s lead product candidate for ulcerative colitis, an estimated $7 billion-plus market that hosts many therapies but few that are effective or don’t come with side effects. It works by tamping down the activity of the enzyme family PDE4, which controls production of cytokines and is implicated in various inflammatory diseases. PALI-2108 is specific to the colon, unlike the PDE4 inhibitorsPDE4 inhibitors approved for other conditions, and is converted into its active form by microbiota in the gut. This may make it less likely than other therapies to cause side effects.
更多内容,请访问原始网站
文中所述内容并不反映新药情报库及其所属公司任何意见及观点,如有版权侵扰或错误之处,请及时联系我们,我们会在24小时内配合处理。
靶点
药物
热门报告
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。




