AACR: To bring CAR-T to solid tumors, Poseida thinks we need to boost conditioning therapy
2024-04-10
临床结果AACR会议细胞疗法免疫疗法上市批准
Preview
来源: FierceBiotech
Poseida found that patients with solid tumors had higher white blood cell counts entering the trial and it wasn’t until the chemotherapy dose was upped to as high as 1,000 mg that they achieved effective lymphodepletion.
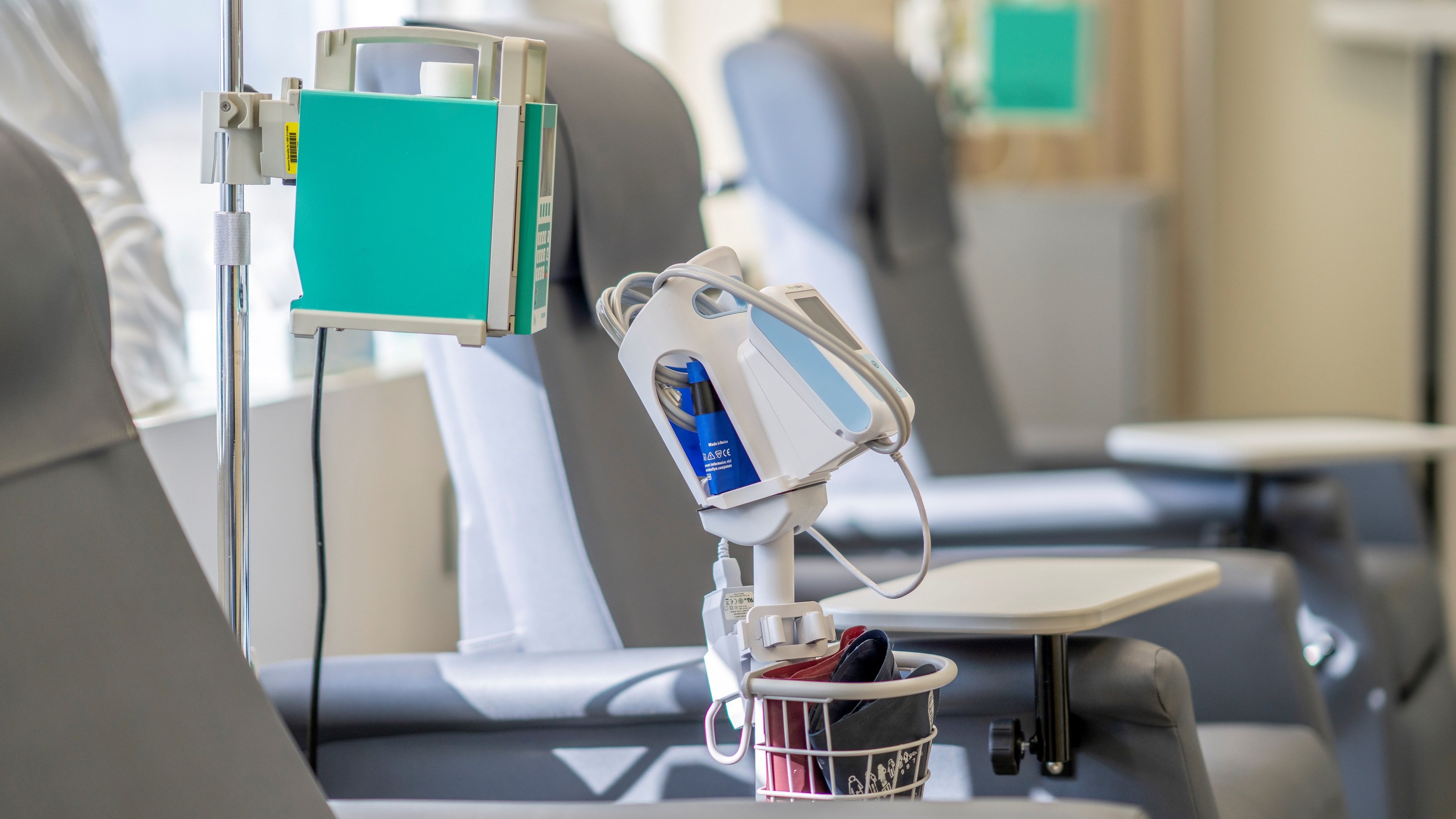
Why haven’t CAR-T therapies cracked into solid tumors yet? Poseida Therapeutics is trying to fill in at least one piece of the puzzle with a new analysis presented at the American Association for Cancer Research annual meeting showing that patients may need a higher dose of lymphodepletion than those with blood cancers.
Many-a-biotech has popped up with bold promises of bringing the technology to solid tumors, too. But these therapies have run into myriad problems because they lack tumor-specific antigens and tend to wither in an immunosuppressive tumor microenvironment.
Poseida’s new retrospective analysis shows that patients with solid tumors might need a higher dose of conditioning chemotherapy to effectively prepare for allogeneic CAR-T treatment.
The company is hoping to add to the collective learning in CAR-T therapy, Rajesh Belani, M.D., told Fierce Biotech on the sidelines of the AACR meeting. Belani is vice president for clinical development at Poseida.
The assumption was that whatever worked for blood cancers would translate over to solid tumors, Belani said. But that doesn’t seem to be the case for the pre-conditioning regimen.
“Nobody has looked at this question,” Belani said.
Blood cancer patients typically receive a dose of 300 mg before starting CAR-T therapy. Poseida found that patients with solid tumors had higher white blood cell counts entering the trial and it wasn’t until the chemotherapy dose was upped to as high as 1,000 mg that they achieved comparable lymphodepletion.
With the higher conditioning dose, patients achieved better CAR-T cell expansion. In the AACR presentation, Poseida said “there was a trend” towards improved expansion of the biotech’s therapy, P-MUC1C-ALLO1, in those who received the 1,000 mg lymphodepletion dose.
Poseida also examined a 500 mg dose but found that the lymphodepletion wasn’t where it needed to be to proceed with CAR-T therapy.
Elsewhere at AACR, Poseida presented cohort data from a subset of patients in a phase 1/2 trial of P-BCMA-ALLO1, which is being developed with Roche in multiple myeloma.
The small dataset included five patients who had previously progressed after treatment with BCMA-targeted therapies such as bispecific T-cell engagers, antibody-drug conjugates (ADCs) or autologous CAR-T. These patients had received as many as seven prior treatments.
One patient’s response deepened to a “very good partial response” after receiving P-BCMA-ALLO1. The patient died about 60 days after therapy due to unrelated events, meaning the depth of response is hard to make sense of, analysts from William Blair noted in a Monday analysis.
Another patient also deepened to a very good partial response but progressed after 80 days. And a third patient has reached the same response that is continuing 120 days after receiving P-BCMA-ALLO1.
Enrollment in the phase 1/2 study will continue with patients who have prior BCMA exposure.
更多内容,请访问原始网站
文中所述内容并不反映新药情报库及其所属公司任何意见及观点,如有版权侵扰或错误之处,请及时联系我们,我们会在24小时内配合处理。
靶点
热门报告
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。