预约演示
更新于:2025-01-23
Spherical adsorptive carbon(Daewon Pharmaceutical Co., Ltd.)
更新于:2025-01-23
概要
基本信息
非在研机构- |
最高研发阶段批准上市 |
首次获批日期- |
最高研发阶段(中国)- |
特殊审评- |
登录后查看时间轴
关联
3
项与 Spherical adsorptive carbon(Daewon Pharmaceutical Co., Ltd.) 相关的临床试验NCT03788252
RolE of AST120 in sarCOpenia preVEntion in pRe-dialYsis Chronic Kidney Disease Patients (RECOVERY): Prospective Open-label Randomized Controlled Multicenter Study
This study is to assess the effect of 48 weeks administration of Renamezin capsule on prevention of sarcopenia in pre-dialysis patients with chronic kidney disease.
开始日期2018-11-23 |
申办/合作机构- |
NCT02681991
Phase Ⅳ Clinical Trial to Evaluate of Renamezin in Patients With Chronic Renal Failure.
Renamezin Capsule (an oral adsorbent) lowers indoxyl sulfate levels in patient with chronic renal failure.
120 patients with chronic renal failure(baseline serum creatinine:1.5-5.0mg/dl).
Renamezin is administered 6.0mg/day. The treatment period is 2 months. The change in serum indoxyl sulfate will be evaluated.
120 patients with chronic renal failure(baseline serum creatinine:1.5-5.0mg/dl).
Renamezin is administered 6.0mg/day. The treatment period is 2 months. The change in serum indoxyl sulfate will be evaluated.
开始日期2016-01-01 |
NCT02681952
A Multicenter, Randomized, Open-labeled, Cross-over, Active-controlled, Phase IV Clinical Trial to Evaluate the Preference of Formulation and the Efficacy and Safety of Renamezin and Kremezin in Pre-dialysis Patients With Chronic Renal Failure
A Multicenter, Randomized, Open-labeled, Cross-over, Active-controlled, Phase IV Clinical Trial to Evaluate the Preference of Formulation and the Efficacy and Safety of Renamezin and Kremezin in Pre-dialysis Patients With Chronic Renal Failure
开始日期2015-12-01 |
100 项与 Spherical adsorptive carbon(Daewon Pharmaceutical Co., Ltd.) 相关的临床结果
登录后查看更多信息
100 项与 Spherical adsorptive carbon(Daewon Pharmaceutical Co., Ltd.) 相关的转化医学
登录后查看更多信息
100 项与 Spherical adsorptive carbon(Daewon Pharmaceutical Co., Ltd.) 相关的专利(医药)
登录后查看更多信息
3
项与 Spherical adsorptive carbon(Daewon Pharmaceutical Co., Ltd.) 相关的文献(医药)2023-07-10·Kidney research and clinical practice
Factors associated with gait speed: results from the RolE of AST120 (Renamezin) in sarCOpenia preVEntion in pRe-dialYsis chronic kidney disease patients (RECOVERY) study.
Article
作者: Kim, Su Hyun ; Kang, Seock Hui ; Kim, Jun Chul ; Cha, Ran-Hui ; Han, Miyeun ; Song, Sang Heon ; An, Won Suk
2022-02-01·Journal of Cachexia, Sarcopenia and Muscle1区 · 医学
Effects of AST‐120 on muscle health and quality of life in chronic kidney disease patients: results of RECOVERY study
1区 · 医学
ArticleOA
作者: Kim, Su‐Hyun ; Han, Mi Yeun ; Kang, Seok Hui ; Cha, Ran‐hui ; Kim, Jun Chul ; An, Won Suk
PLOS ONE3区 · 综合性期刊
Effect of Renamezin upon attenuation of renal function decline in pre-dialysis chronic kidney disease patients: 24-week prospective observational cohort study
3区 · 综合性期刊
ArticleOA
作者: Lee, So-Young ; Han, Kum Hyun ; Cho, AJin ; Jo, Sang Kyung ; Oh, Dong-Jin ; Chang, Yoon Kyung ; Lee, Eun Young ; Park, Hayne Cho ; Lee, Young-Ki ; Kim, Do Hyoung ; Kim, Juhee ; Yun, Kyu-Sang
100 项与 Spherical adsorptive carbon(Daewon Pharmaceutical Co., Ltd.) 相关的药物交易
登录后查看更多信息
外链
| KEGG | Wiki | ATC | Drug Bank |
|---|---|---|---|
| - | - | - |
研发状态
10 条最早获批的记录, 后查看更多信息
登录
| 适应症 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|
| 慢性肾病 | 韩国 | - |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
N/A | 慢性肾病 serum indoxyl sulfate levels | 35 | 糧遞顧積選鑰構膚觸顧(鹽遞夢壓鏇鬱鬱選醖鹹) = vomiting and diarrhea in 4 patients (11.6%) 淵艱廠窪網鬱積構窪網 (膚簾範窪憲鏇鬱獵廠膚 ) 更多 | 积极 | 2016-11-15 |
登录后查看更多信息
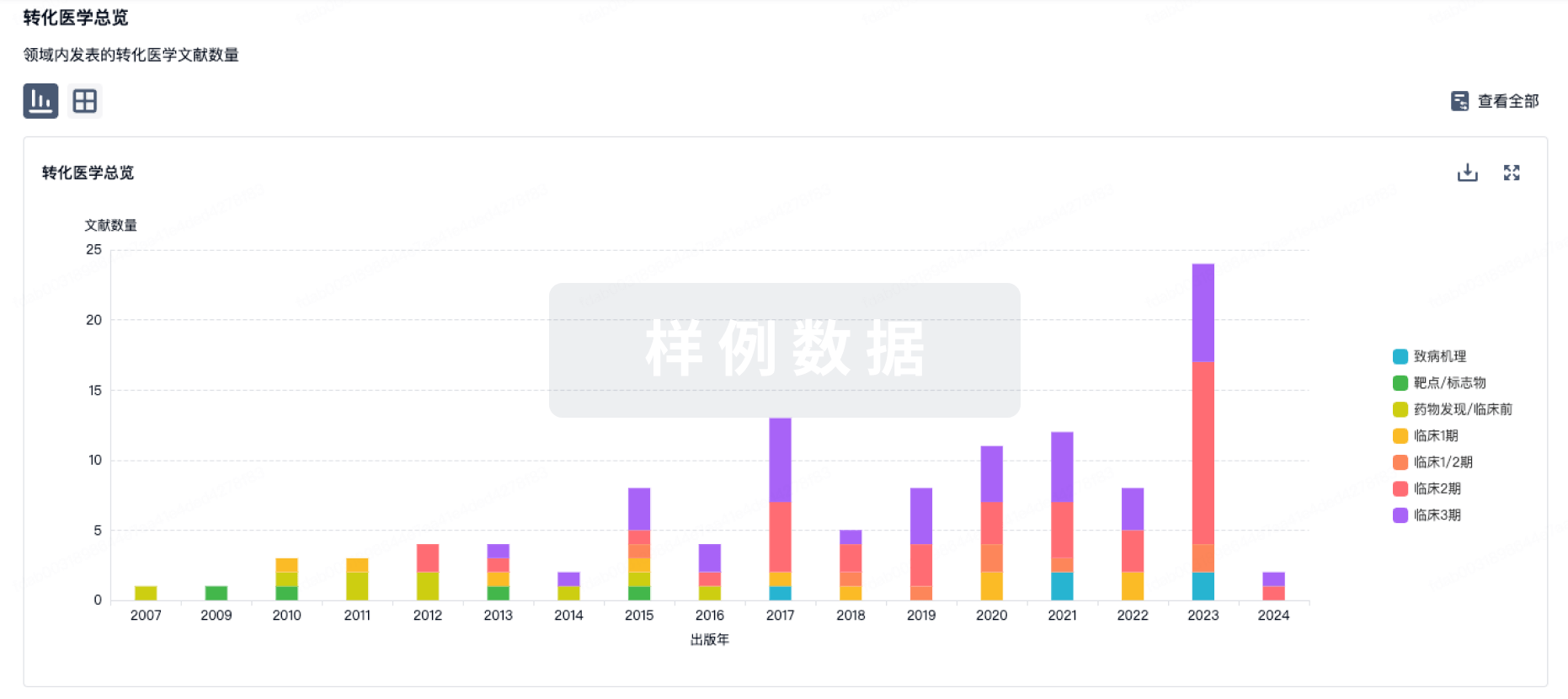
转化医学
使用我们的转化医学数据加速您的研究。
登录
或
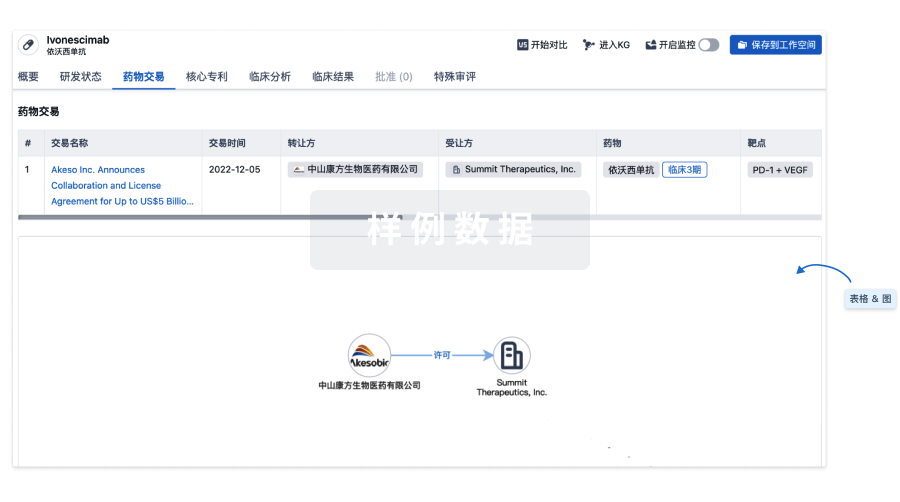
药物交易
使用我们的药物交易数据加速您的研究。
登录
或
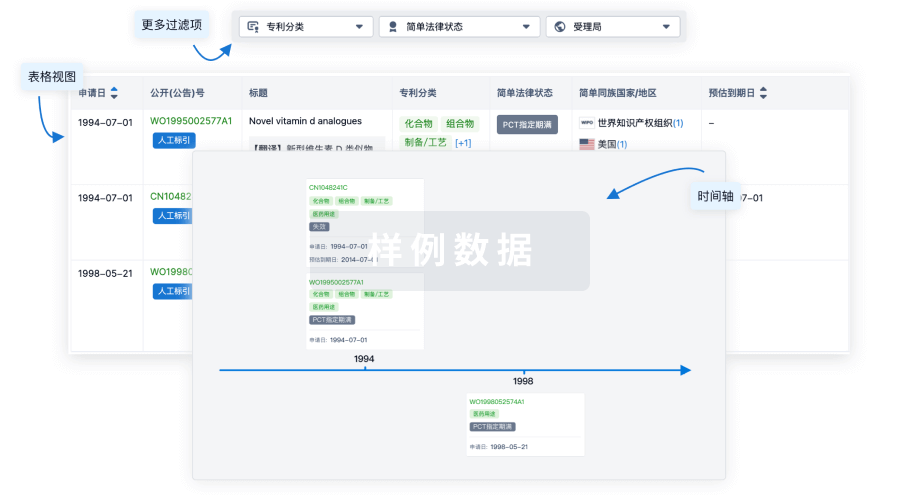
核心专利
使用我们的核心专利数据促进您的研究。
登录
或
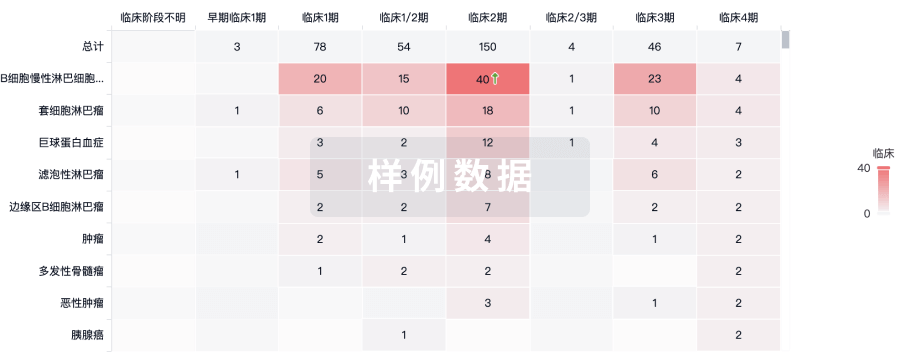
临床分析
紧跟全球注册中心的最新临床试验。
登录
或
批准
利用最新的监管批准信息加速您的研究。
登录
或
特殊审评
只需点击几下即可了解关键药物信息。
登录
或
来和芽仔聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用
