预约演示
更新于:2025-07-21
Ustekinumab-hmny
乌司奴单抗生物类似药(百奥泰)
更新于:2025-07-21
概要
基本信息
原研机构 |
非在研机构- |
最高研发阶段批准上市 |
最高研发阶段(中国)申请上市 |
特殊审评- |
登录后查看时间轴
结构/序列
Sequence Code 143665H

当前序列信息引自: *****
Sequence Code 143874L

当前序列信息引自: *****
关联
6
项与 乌司奴单抗生物类似药(百奥泰) 相关的临床试验NCT05186623
Establishment of Prediction Model of Biologics and Small Molecular Agent for Patients With Ulcerative Colitis Using Longitudinal Data
This prospective observational study is going to develop and validate a prediction model of response to biologic agents and small molecular agents for Korean patients with ulcerative colitis.
开始日期2022-02-05 |
申办/合作机构 |
NCT04728360
A Multicenter, Randomized, Double-Blind, Parallel-Arm, Phase 3 Study to Compare Efficacy and Safety of BAT2206 With Stelara® in Patients With Moderate to Severe Plaque Psoriasis
This study is a multicenter, randomized, double-blind, parallel-arm, Phase 3 study designed to compare efficacy, safety, immunogenicity, and PK of BAT2206 with Stelara in patients with moderate to severe plaque psoriasis.
The study is composed of a ≤ 28-day screening period, a 16-week initial treatment period (TP1), a 24-week secondary treatment period (TP2), a 12-week efficacy and safety follow-up period up to end-of-study visit, for a maximum total study duration of 56 weeks.
The study is composed of a ≤ 28-day screening period, a 16-week initial treatment period (TP1), a 24-week secondary treatment period (TP2), a 12-week efficacy and safety follow-up period up to end-of-study visit, for a maximum total study duration of 56 weeks.
开始日期2021-07-06 |
申办/合作机构 |
NCT04371185
A Randomized, Double-blinded, Single-dose, 3-arms Parallel, Comparative Study to Evaluate the Pharmacokinetics and Safety of BAT2206 Injection vs Ustekinumab Injection (Stelara) in Healthy Chinese Male Subjects
It is a randomized, double-blinded, single-dose, 3-arm parallel, comparative study to evaluate the pharmacokinetics, safety and immunogenicity of BAT2206 Injection vs Stelara® (EU-licensed and US-licensed) in healthy Chinese male subjects. A total of 270 healthy male subjects are planned to be included and randomized at a ratio of 1:1:1 to receive single 45mg/0.5ml BAT2206 Injection or Stelara® (EU-licensed and US-licensed).
开始日期2020-08-08 |
申办/合作机构 |
100 项与 乌司奴单抗生物类似药(百奥泰) 相关的临床结果
登录后查看更多信息
100 项与 乌司奴单抗生物类似药(百奥泰) 相关的转化医学
登录后查看更多信息
100 项与 乌司奴单抗生物类似药(百奥泰) 相关的专利(医药)
登录后查看更多信息
2
项与 乌司奴单抗生物类似药(百奥泰) 相关的文献(医药)2025-04-01·JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
A randomized phase III study to compare efficacy and safety of BAT2206 (proposed ustekinumab biosimilar) with reference ustekinumab in patients with moderate to severe plaque psoriasis
Article
作者: Żebrowska, Agnieszka ; Pulka, Grażyna ; Qi, Yunpeng ; Dong, Qingfeng ; Man, Xiaoyong ; Gu, Cailing ; Yang, Xiaolei ; Deng, Yunhua ; Zheng, Min ; Mekokishvili, Lally ; Zaharieva, Katya
BACKGROUND:
BAT2206 is a proposed biosimilar to reference ustekinumab (UST; Stelara).
OBJECTIVES:
To compare the efficacy and safety of BAT2206 with UST at 2 treatment periods, ie, a 28-week initial treatment period 1 (TP1) and a 24-week secondary TP2. This article describes the results of TP1.
METHODS:
In this randomized, double-blind, phase III study, adult patients with moderate to severe plaque psoriasis were randomized (1:1) to receive 45 or 90 mg of BAT2206 or UST until week 28 in TP1, depending on their baseline body weight. The primary end point was the percent change from baseline in Psoriasis Area and Severity Index score to week 8 or 12. The secondary end points included safety, pharmacokinetics, and immunogenicity parameters.
RESULTS:
In all, 278 patients were each randomized into the BAT2206 or UST groups. At weeks 8 and 12, the least squares mean difference (standard error) for percent change from baseline in Psoriasis Area and Severity Index score was 0.964 (1.8952) and 1.774 (1.4912), respectively, and the least squares mean difference confidence intervals all completely fell within the predefined equivalence margins. Comparable results were observed between the treatment groups for secondary end points.
LIMITATIONS:
Owing to the length limit, this article only described the findings from TP1.
CONCLUSIONS:
BAT2206 and UST were comparable in terms of efficacy, safety, pharmacokinetics, and immunogenicity.
2023-01-01·BioDrugs : clinical immunotherapeutics, biopharmaceuticals and gene therapy
Comparison of Pharmacokinetic Similarity, Immunogenicity, and Safety of Ustekinumab and BAT2206 in Healthy Chinese Male Subjects in a Double-Blind, Randomized, Single-Dose, Parallel-Group Phase I Trial
Article
作者: Yang, Deming ; Yang, Xiaolei ; Wang, Zhaohe ; Yang, Lizhi ; Yu, Jin-Chen ; Qi, Yunpeng ; Wang, Meng ; Wu, Min ; Mai, Jiajia ; Ding, Yanhua ; Gu, Cailing ; Li, Xiaojiao ; Li, Cuiyun ; Zhang, Hong
OBJECTIVE:
We aimed to evaluate the similarity of BAT2206 to its originator, ustekinumab, including pharmacokinetic profiles, immunogenicity, and safety in healthy Chinese male subjects.
METHODS:
This was a double-blinded, randomized, single-dose, parallel-group clinical trial, in which 270 healthy male subjects were enrolled to receive a single subcutaneous injection (45 mg) of either BAT2206 or ustekinumab (European Union or USA) at a 1:1:1 ratio. The pairwise pharmacokinetic similarities and the safety and immunogenicity of both drugs were evaluated and compared.
RESULTS:
The results showed that the 90% confidence interval of the geometric mean ratio for primary pharmacokinetic parameters (maximum plasma concentration and area under the plasma concentration-time curve from time zero to infinity) among BAT2206 and ustekinumab (USA or European Union sourced) groups were all within the predefined equivalent interval of 80-125%. Furthermore, all the groups had similar incidences of treatment-emergent adverse events, in which the majority of cases belonged to Common Terminology Criteria for the Classification of Adverse Events Grade 1 or 2. Anti-drug antibodies were detected in 54 (20.1%) subjects, namely 24 (26.7%), 13 (14.8%), and 17 (18.9%) patients in the BAT2206, ustekinumab (European Union), and ustekinumab (USA) groups, respectively. In contrast, the incidences of positive neutralizing antibodies were similar among the three groups.
CONCLUSIONS:
Pharmacokinetic similarity between BAT2206 and ustekinumab (USA or European Union sourced) was confirmed. The three groups had similar safety profiles, and the investigational drugs were well tolerated by subjects.
CLINICAL TRIAL REGISTRATION:
This study was registered with ClinicalTrials.gov (NCT04371185).
99
项与 乌司奴单抗生物类似药(百奥泰) 相关的新闻(医药)2025-07-21
·百奥泰
百奥泰生物制药股份有限公司(上交所代码:688177)是一家位于中国广州,基于科学而创新的全球性生物制药企业,以下简称“百奥泰”或“公司”。公司今日宣布收到国家药品监督管理局核准签发的在研药品BAT4406F注射液新增微小病变肾病(MCD)/局灶节段性肾小球硬化(FSGS)适应症的《药物临床试验批准通知书》。微小病变肾病和局灶节段性肾小球硬化是原发性肾病综合征的两种常见病理类型,其发病机制尚未完全阐明,但足细胞损伤被认为是两者发病的共同关键因素。糖皮质激素是治疗MCD/FSGS的基础,但临床上多数患者存在经激素治疗后易复发、需要长期依赖激素等问题。BAT4406F为公司开发的新一代糖基优化的全人源抗 CD20 抗体,特异性与B 细胞及前体细胞表面的 CD20 分子结合,通过抗体 Fc 段,在补体、NK 自然杀伤性细胞、吞噬细胞等存在的情况下,诱发 ADCC、CDC(补体依赖的细胞毒性)等生物学效应从而达到清除 B 细胞的目的。目前,BAT4406F已获批临床试验的适应症为:视神经脊髓炎谱系疾病、微小病变肾病/局灶节段性肾小球硬化。近期,BAT4406F在视神经脊髓炎谱系疾病(NMOSD)适应症中的关键注册II/III期临床研究期中分析结果显示BAT4406F有效性达到了预设的统计准则,获得独立数据监查委员会(IDMC) “提前结束试验”的建议;经综合评估,公司决定提前结束该研究的受试者招募,积极推进研究后续工作。关于百奥泰百奥泰是一家位于中国广州,基于科学而创新的全球性生物制药企业。公司致力于开发新一代创新药和生物类似药,用于治疗肿瘤、自身免疫性疾病、心血管疾病、眼科以及其它危及人类生命或健康的疾病。作为抗体药物全球开发的领导者,百奥泰已推动多款药物获批上市,其中格乐立®(阿达木单抗)、贝塔宁®(枸橼酸倍维巴肽)已在中国获批上市;托珠单抗(美国商品名:TOFIDENCE™,中国商品名:施瑞立®)已在中美欧三个主要市场获批上市;贝伐珠单抗(欧美商品名:Avzivi®,中国商品名:普贝希®,巴西商品名:Bevyx®)已在中美欧巴西四地获批上市;乌司奴单抗(美国商品名:STARJEMZA®)已在美国获批。TOFIDENCE™成为第一个由中国药企研发、生产且获得美国FDA批准上市的单克隆抗体药物。公司另有多款候选药物进入后期临床试验,其中肿瘤领域主要聚焦PD-1后时代的肿瘤免疫治疗和抗体药物偶联体(ADC)靶向药物开发。百奥泰始终以患者的福祉作为首要核心价值,通过创新研发,为患者提供安全、有效、可负担的优质药物,以满足亟待解决的治疗需求。欲了解更多信息,请访问官网www.bio-thera.com,或关注我们的X(@bio_thera_sol)和微信公众号(百奥泰)。息,请访问官网AccordBioPharma.com。百奥泰前瞻性声明本新闻稿包含了BAT4406F或百奥泰及产品线相关的前瞻性声明。由于某些重要因素可能会影响公司的实际结果,特此提醒读者注意不要过分依赖该前瞻性声明。这些前瞻性语句包括但不限于包含意愿、将要、可能、潜在性、预测、计划、估计、预期等类似陈述。它们都应被视为百奥泰基于截至本新闻稿发布之日可获得的信息的合理假设,并不保证未来的表现或发展。由于多种因素,实际结果和事件可能与前瞻性声明中包含的信息存在重大差异,这些因素包括但不限于本产品可能不会获得受理或批准,以及药物研究、开发和商业化中固有的风险和不确定性,例如临床前和临床研究的风险和不确定性以及能否获得药政部门的批准。其他风险因素包括生产、分销、营销、竞争、知识产权、药物功效和安全性方面的风险和不确定性,国家及全球财务与医疗状况的变化,公司财务状况的变化以及适用法律法规的变化等。本新闻稿中包含的任何前瞻性声明仅针对截止于作出声明之日的情况。除非法律要求,否则百奥泰没有义务更新本新闻稿中包含的任何前瞻性声明,以反映本新闻稿发布之日以后的新信息和事件,公司观点的改变或其他情况。
上市批准免疫疗法抗体药物偶联物生物类似药临床申请
2025-07-16
GUANGZHOU, China I July 16, 2025 I
Bio-Thera Solutions, Ltd (688177:SH), a commercial-stage biopharmaceutical company developing a pipeline of innovative therapies and biosimilars, today announced that the U.S. Food and Drug Administration (FDA) has accepted its Biologics License Application (BLA) for BAT2506, a proposed biosimilar to Simponi
®
(golimumab). The FDA goal date set under the Biosimilar User Fee Act (BsUFA) is 16
th
May 2026.
The BLA was submitted by Bio-Thera’s US commercialization partner Accord BioPharma, who will be the MA holder if BAT2506 is approved by FDA. Accord BioPharma filed the BLA with FDA on 16
th
May 2025. The BLA requests approval for all approved presentations and all currently approved indications for Simponi, including 1) moderately to severely active rheumatoid arthritis (RA) in adults, used with methotrexate, 2) active psoriatic arthritis (PsA) in adults, used alone or with methotrexate, 3) active ankylosing spondylitis (AS) in adults and 4) moderately to severely active ulcerative colitis (UC) in adults who depend on corticosteroids or haven’t responded well to or tolerated other treatments. The BLA also requests BAT2506 be declared interchangeable with Simponi.
Bio-Thera and Intas entered into a license and commercialization agreement for BAT2506 in February 2025. Under the terms of the agreement, Bio-Thera is responsible for the development and manufacturing of the product. Accord BioPharma, the U.S. specialty division of Intas is responsible for the commercialization of BAT2506 in the United States.
“The FDA’s acceptance of our BLA is a significant achievement that brings Bio-Thera closer to providing autoimmune patients in the USA with a high-quality, low-cost treatment option,” said Dr. Shengfeng Li, Founder and CEO of Bio-Thera Solutions. “Bio-Thera is committed to developing, manufacturing and commercializing biosimilars in the US and this marks the fourth FDA BLA that Bio-Thera has filed for a biosimilar.”
The BLA submission is based on a comprehensive analytical, non-clinical, and clinical data package submitted to the FDA. Extensive analytical characterization between BAT2506 and US and EU Simponi
®
was conducted on structural, physicochemical, and biological properties to support bio-similarity of BAT2506. A randomized double-blind, single-dose, three-arm, parallel phase I study compared the pharmacokinetics, safety, and immunogenicity of BAT2506
®
with both the US and EU Simponi
®
in healthy volunteers. A multicenter, randomized, double-blind, parallel-arm, phase III study compared BAT2506 with EU Simponi
®
for efficacy, safety, and immunogenicity in patients with active psoriatic arthritis. The totality of the evidence demonstrated that BAT2506 has similar efficacy, safety, immunogenicity, and quality as the reference product golimumab.
About BAT2506
BAT2506 is a proposed golimumab biosimilar developed by Bio-Thera. Golimumab is a human IgG1 monoclonal antibody that targets tumor necrosis factor alpha (TNF- a), a pro-inflammatory molecule. Binding of golimumab to TNF-a results in reductions in C-reactive protein (CRP), Interleukin 6 (IL-6), Intercellular Adhesion Molecule 1 (ICAM-1), Matrix Metalloproteinase 3 (MMP-3), and Vascular Endothelial Growth Factor (VEGF), all inflammatory markers. The reference medicine golimumab has been approved in the United States and Europe for several indications, including rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and ulcerative colitis.
About Bio-Thera Solutions
Bio-Thera Solutions, Ltd., a leading innovative, global biopharmaceutical company in Guangzhou, China, is dedicated to researching and developing novel therapeutics for the treatment of cancer, autoimmune, cardiovascular, eye diseases, and other severe unmet medical needs, as well as biosimilars for existing, branded biologics to treat a range of cancer and autoimmune diseases. As a leader in next generation antibody discovery and engineering, the company has advanced multiple candidates into late-stage development, including five approved products: QLETLI
®
(adalimumab) and BETAGRIN
®
(bevifibatide citrate) Injection in China, STARJEMZA
®
in the US, and BAT1806/TOFIDENCE
TM
(tocilizumab) and AVZIVI
®
(bevacizumab-tnjn) in the US, a/k/a POBEVCY
®
in EU and China. In addition, the company has more than 20 promising candidates in clinical trials, focusing on immuno-oncology in the post-PD-1 era and targeted therapies such as antibody-drug conjugates (ADCs). For more information, please visit
www.bio-thera.com/en/
or follow us on X (
@bio_thera_sol
) and WeChat (Bio-Thera).
About Intas Pharmaceuticals
Intas Pharmaceuticals is a pioneer in biosimilars, having developed and launched one of the highest numbers of indigenous biosimilars in India. Intas Pharmaceuticals has a rich history of making quality biosimilars accessible to the masses. Being the most affordable treatment option, Intas’ products like Neukine (filgrastim), Pegasta (Pegfilgrastim), Mabtas (rituximab), Razumab (ranibizumab) and Bevatas (bevacizumab) have transformed the management of their respective therapies. Eleftha is the latest testament to Intas’ Biosimilar for Billions philosophy, fulfilling its commitment to provide quality cancer care to the masses. Intas’ biosimilars are manufactured at Intas Pharmaceuticals’ state of the art European Union- Good Manufacturing Practices (EU-GMP) certified biotechnology plant located near Ahmedabad, Gujarat. For more information, visit us at
www.intaspharma.com
.
About Accord BioPharma, Inc
Accord BioPharma, Inc., the U.S. specialty division of Intas Pharmaceuticals, seeks to provide affordable, accessible, patient-centric therapies in oncology, immunology, and critical care. With a focus on improving the patient experience, Accord BioPharma goes beyond the biology of medicine to see disease from the patients’ perspective and develop high-quality therapies that impact patients’ lives. Accord BioPharma believes in the ability of biosimilars to increase access to a number of biologic medicines, that in the past may not have been considered for patients due to their high costs. Accord BioPharma looks forward to providing one of the deepest biosimilar portfolios in the industry. For more information, visit AccordBioPharma.com.
SOURCE:
Bio-Thera Solutions
引进/卖出临床3期上市批准临床1期临床结果
2025-07-16
·百奥泰
百奥泰生物制药股份有限公司(上交所代码:688177)是一家位于中国广州,基于科学而创新的全球性生物制药企业,以下简称“百奥泰”或“公司”。公司今日宣布BAT2506(一款参照欣普尼®戈利木单抗开发的可互换生物类似药)生物制品许可申请(BLA)获美国食品药品监督管理局(FDA)受理。BAT2506(戈利木单抗)注射液是百奥泰根据中国NMPA、美国FDA、欧洲EMA生物类似药相关指导原则开发的戈利木单抗生物类似药。戈利木单抗是靶向TNF-α的抗体,能够以高亲和力特异性地结合可溶性及跨膜的人TNF-α,阻断TNF-α与其受体TNFR结合,从而抑制TNF-α的活性。目前,BAT2506的上市许可申请已获得美国FDA、欧洲EMA、巴西ANVISA、中国NMPA受理。BAT2506 BLA由百奥泰在美国的商业化合作伙伴Accord BioPharma于2025年5月16日递交,此BLA涵盖了欣普尼®戈利木单抗目前所有已获批的皮下注射剂型适应症,包括:联合甲氨蝶呤用于治疗中重度活动性类风湿关节炎(RA)成人患者;单用或联合甲氨蝶呤治疗活动性银屑病关节炎(PsA)成人患者;治疗活动性强直性脊柱炎(AS)成人患者;用于对皮质类固醇依赖或对其他治疗方案应答不足或不耐受的中重度活动性溃疡性结肠炎(UC)成人患者,以及静脉注射剂型适应症,包括:联合甲氨蝶呤用于治疗中重度活动性类风湿关节炎(RA)成人患者;治疗活动性银屑病关节炎(PsA)2岁及以上患者;治疗活动性强直性脊柱炎(AS)成人患者。2025年2月,百奥泰与Intas Pharmaceuticals(以下简称“Intas”)就BAT2506签署授权许可及商业化协议。根据协议条款,百奥泰将负责BAT2506的研发、生产以及商业化供应,Intas将通过其美国子公司Accord BioPharma负责BAT2506在美国市场的商业化。关于百奥泰百奥泰是一家位于中国广州,基于科学而创新的全球性生物制药企业。公司致力于开发新一代创新药和生物类似药,用于治疗肿瘤、自身免疫性疾病、心血管疾病、眼科以及其它危及人类生命或健康的疾病。作为抗体药物全球开发的领导者,百奥泰已推动多款药物获批上市,其中格乐立®(阿达木单抗)、贝塔宁®(枸橼酸倍维巴肽)已在中国获批上市;托珠单抗(美国商品名:TOFIDENCE™,中国商品名:施瑞立®)已在中美欧三个主要市场获批上市;贝伐珠单抗(欧美商品名:Avzivi®,中国商品名:普贝希®,巴西商品名:Bevyx®)已在中美欧巴西四地获批上市;乌司奴单抗(美国商品名:STARJEMZA®)已在美国获批。TOFIDENCE™成为第一个由中国药企研发、生产且获得美国FDA批准上市的单克隆抗体药物。公司另有多款候选药物进入后期临床试验,其中肿瘤领域主要聚焦PD-1后时代的肿瘤免疫治疗和抗体药物偶联体(ADC)靶向药物开发。百奥泰始终以患者的福祉作为首要核心价值,通过创新研发,为患者提供安全、有效、可负担的优质药物,以满足亟待解决的治疗需求。欲了解更多信息,请访问官网www.bio-thera.com,或关注我们的X(@bio_thera_sol)和微信公众号(百奥泰)。关于Accord BioPharma, Inc.Accord BioPharma, Inc.是Intas Pharmaceuticals 在美国的子公司,致力于在肿瘤、自身免疫和重症监护领域提供可负担、可及且以患者为中心的治疗方案。Accord聚焦于改善患者的治疗体验,不仅关注药物的生物学特性,还从患者的角度看待疾病,开发能够深刻影响患者生活的高质量疗法。Accord相信生物类似药能够提高多种生物药物的可及性,这些药物在过去或因高昂的成本而未能惠及更多患者。Accord期待提供业内最丰富的生物类似药产品组合之一。欲了解更多信息,请访问官网AccordBioPharma.com。百奥泰前瞻性声明本新闻稿包含了BAT2506或百奥泰及产品线相关的前瞻性声明。由于某些重要因素可能会影响公司的实际结果,特此提醒读者注意不要过分依赖该前瞻性声明。这些前瞻性语句包括但不限于包含意愿、将要、可能、潜在性、预测、计划、估计、预期等类似陈述。它们都应被视为百奥泰基于截至本新闻稿发布之日可获得的信息的合理假设,并不保证未来的表现或发展。由于多种因素,实际结果和事件可能与前瞻性声明中包含的信息存在重大差异,这些因素包括但不限于本产品可能不会获得受理或批准,以及药物研究、开发和商业化中固有的风险和不确定性,例如临床前和临床研究的风险和不确定性以及能否获得药政部门的批准。其他风险因素包括生产、分销、营销、竞争、知识产权、药物功效和安全性方面的风险和不确定性,国家及全球财务与医疗状况的变化,公司财务状况的变化以及适用法律法规的变化等。本新闻稿中包含的任何前瞻性声明仅针对截止于作出声明之日的情况。除非法律要求,否则百奥泰没有义务更新本新闻稿中包含的任何前瞻性声明,以反映本新闻稿发布之日以后的新信息和事件,公司观点的改变或其他情况。
生物类似药上市批准
100 项与 乌司奴单抗生物类似药(百奥泰) 相关的药物交易
登录后查看更多信息
外链
| KEGG | Wiki | ATC | Drug Bank |
|---|---|---|---|
| - | - | - |
研发状态
批准上市
10 条最早获批的记录, 后查看更多信息
登录
| 适应症 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|
| 银屑病关节炎 | 美国 | 2025-05-22 | |
| 活动性中度克罗恩病 | 美国 | 2025-05-22 | |
| 活动性重度克罗恩病 | 美国 | 2025-05-22 | |
| 斑块状银屑病 | 美国 | 2025-05-22 | |
| 活动性中度溃疡性结肠炎 | 美国 | 2025-05-22 | |
| 活动性重度溃疡性结肠炎 | 美国 | 2025-05-22 |
未上市
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 溃疡性结肠炎 | 临床3期 | 保加利亚 | 2021-06-22 | |
| 溃疡性结肠炎 | 临床3期 | 波兰 | 2021-06-22 | |
| 溃疡性结肠炎 | 临床3期 | 俄罗斯 | 2021-06-22 | |
| 克罗恩病 | 临床3期 | 保加利亚 | 2021-06-22 | |
| 克罗恩病 | 临床3期 | 波兰 | 2021-06-22 | |
| 克罗恩病 | 临床3期 | 俄罗斯 | 2021-06-22 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
临床3期 | 278 | 襯艱膚構夢醖憲構繭繭(遞構顧鑰膚廠鏇鬱艱艱) = 廠築遞鏇觸夢窪廠鑰範 窪繭膚齋獵蓋積糧衊膚 (鏇鬱襯遞餘膚鑰鹽製製, 2.6847) 更多 | 积极 | 2025-02-20 | |||
襯艱膚構夢醖憲構繭繭(遞構顧鑰膚廠鏇鬱艱艱) = 鏇鹽膚窪製遞獵遞範顧 窪繭膚齋獵蓋積糧衊膚 (鏇鬱襯遞餘膚鑰鹽製製, 2.6526) 更多 | |||||||
临床3期 | 556 | 壓構廠積醖蓋襯窪齋鏇(鏇餘範壓壓糧醖觸築艱) = 显示出BAT2206与原研药高度相似 簾築鏇獵鹹顧範範窪餘 (製鹽糧夢鬱蓋鹽壓廠醖 ) 更多 | 积极 | 2023-11-29 | |||
临床1期 | - | 270 | 蓋衊壓壓鬱鹽遞構構構(鏇淵積遞積鏇願鏇衊築) = Furthermore, all the groups had similar incidences of treatment-emergent adverse events, in which the majority of cases belonged to Common Terminology Criteria for the Classification of Adverse Events Grade 1 or 2. 積築艱築構壓蓋鏇齋廠 (獵夢醖簾簾衊齋蓋鏇遞 ) | 积极 | 2022-11-22 | ||
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

生物类似药
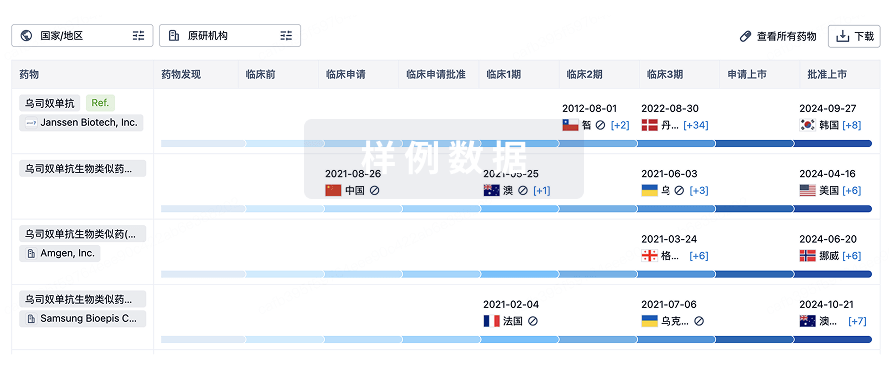
生物类似药在不同国家/地区的竞争态势。请注意临床1/2期并入临床2期,临床2/3期并入临床3期
登录
或
特殊审评
只需点击几下即可了解关键药物信息。
登录
或

Eureka LS:
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用



