6
项与 Trastuzumab Pamirtecan 相关的临床试验A Phase III, Randomized, Multi-site, Open-label Trial of BNT323/DB-1303 Versus Investigator's Choice of Chemotherapy in Previously Treated Patients With HER2- Expressing Recurrent Endometrial Cancer
The goal of this clinical study is to assess the efficacy of BNT323/DB-1303 compared with investigator's choice of chemotherapy in terms of progression-free survival (PFS) by blinded independent central review (BICR) in the endometrial cancer population with prior immune checkpoint inhibitor (ICI) treatment.
A Phase I/II, Multi-site, Open-label, Two-part Trial to Evaluate the Efficacy, Safety, and Pharmacokinetics of BNT323 in Combination With BNT327 in Participants With Advanced Breast Cancer
This is a Phase I/II, multi-site, open-label, two-part study designed to evaluate the efficacy, safety, optimized dose and contribution of components of BNT323 in combination with BNT327 in participants with hormone receptor-positive (HR+) or hormone receptor-negative (HR-), Human epidermal growth factor receptor (HER)2-positive, HER2-low (immunohistochemistry [IHC] 1+ or IHC 2+/in situ hybridization -), HER2-ultralow (IHC 0, with membrane staining) or HER2-null breast cancer (BC), or triple-negative breast cancer (TNBC).
A Phase III, Multicenter, Open-label, Randomized Study to Compare DB-1303 Versus T-DM1 in Patients With HER2-positive Unresectable/Metastatic Breast Cancer Who Have Been Treated With Trastuzumab and a Taxane (Dynasty-Breast01)
This study is designed to compare efficacy and safety of DB-1303/BNT323 versus T-DM1 in HER2-positive, unresectable and/or metastatic breast cancer patients previously treated with trastuzumab and taxane.
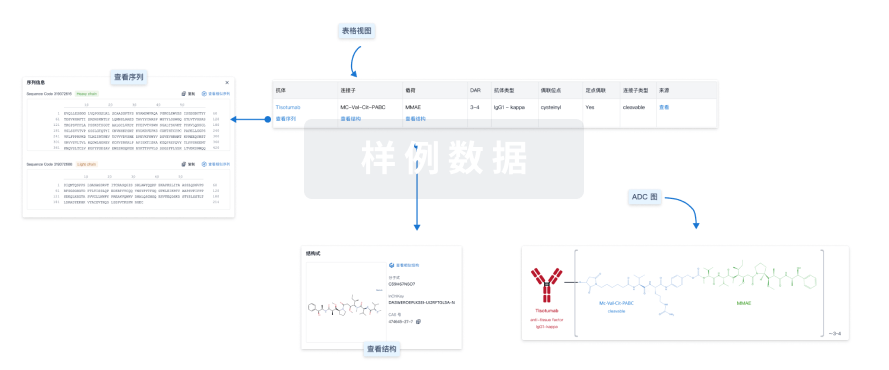
100 项与 Trastuzumab Pamirtecan 相关的临床结果
100 项与 Trastuzumab Pamirtecan 相关的转化医学
100 项与 Trastuzumab Pamirtecan 相关的专利(医药)
251
项与 Trastuzumab Pamirtecan 相关的新闻(医药)点击蓝字
关注我们
本
期
看
点
肿瘤创新药的投资进入到资本异常谨慎阶段,肿瘤创新药的研发早就有着“与上帝掷骰子”的称谓,最为稳妥的成药之路在于机制、靶点已经得到验证过的药物形式的迭代升级,Her2就是典型的代表。
HER2靶点在肿瘤治疗中的地位日益凸显,新一代抗体偶联药物(ADC)不断涌现。近日,恒瑞医药自主研发的注射用瑞康曲妥珠单抗(SHR-A1811)获国家药监局上市批准。国内外针对Her2 ADC产品已经上市和在研的产品众多,恒瑞的瑞康曲妥珠单抗数据对比如何?能否重塑Her2 抗肿瘤治疗格局?
本期内容
01
恒瑞丨Her2 ADC丨进展汇总
02
国内外HER2 ADC产品对比
03
恒瑞ADC研发节奏与差异化策略
04
总结与展望
【01 恒瑞丨Her2 ADC丨进展汇总】
2025年9月17日,根据CDE官网显示,恒瑞医药递交了HER2 ADC药物注射用瑞康曲妥珠单抗(SHR-A1811)的第二项上市申请,推测适应症为治疗既往接受过一种或一种以上抗HER2药物治疗的局部晚期或转移性HER2阳性成人乳腺癌患者。
早在2025年5月29日,恒瑞医药自主研发的注射用瑞康曲妥珠单抗(SHR-A1811)获国家药监局上市批准,适应症为用于治疗存在HER2(ERBB2)激活突变且既往接受过至少一种系统治疗的不可切除的局部晚期或转移性非小细胞肺癌(NSCLC)成人患者。
来源:CDE官网
此次附条件获批是基于上海市胸科医院陆舜教授牵头开展的关键性HORIZON-Lung研究。
除了肺癌和乳腺癌意外以外,恒瑞医药也在CDE药物临床试验登记与信息公示平台网站上,登记了一项HER2 ADC药物SHR-A1811,联合PD-L1抗体阿得贝利单抗以及化疗,对比曲妥珠单抗联合化疗和帕博利珠单抗的一线标准疗法,在不可切除局部晚期或转移性未经治疗胃或胃食管结合部腺癌患者中,启动一项随机、多中心、开放的III期临床研究CTR20253128,这也是SHR-A1811启动第9项三期临床试验。
如此众多的三期临床试验的布局,也是恒瑞坚定在ADC赛道远航的信心说在。
02 国内外HER2 ADC产品对比
下表对比了国内外主要HER2靶向ADC产品的机制、适应症和关键临床数据:
从对比表可见:国际上Enhertu率先大规模布局HER2+乳腺/胃癌,并扩展到HER2低表达及突变NSCLC(全球8项FDA突破性疗法认定);泽尼达妥单抗作为首款HER2双特异性抗体,获批用于HER2+胆道癌,显示ORR逾50%。国内方面,维迪西妥单抗(RC48/Aidixi)已批准用于HER2+胃癌,并在尿路上皮癌等肿瘤中显示良好疗效;DB-1303聚焦HER2+/HER2低表达实体瘤,技术平台独特,已进入临床高位阶段。相比之下,瑞康曲妥珠单抗区别于上述以HER2过表达为主的布局,首创将HER2突变NSCLC作为主要攻关方向,临床表现出优异优势,为这一新靶标患者带来了突破性方案。
【03 恒瑞ADC研发节奏与差异化策略】
恒瑞在ADC领域的研发节奏显著加快,瑞康曲妥珠单抗自上市以来短期内即获CDE多次突破性和优先审评认定,体现出审评快速通道优势。据媒体报道,该药2025年3月第8次被列入拟突破性治疗品种名单。恒瑞的布局策略也颇具差异化:不仅在乳腺癌、胃癌等传统适应症开展研究,更积极开拓HER2突变型NSCLC、宫颈癌等新领域,形成与其他厂商的互补。从技术路线看,恒瑞载荷药物为拓扑异构酶Ⅰ抑制剂,与Enhertu采用类似作用机制(均为拓扑抑制)不同于RC48的MMAE载荷;在抗体选择上,恒瑞采用曲妥珠单抗为骨架,与DB-1303和Enhertu类似,而RC48和Zanidatamab则使用新型抗HER2抗体。总体而言,恒瑞依托本土创新优势,将HER2 ADC研发与临床需求紧密结合,其“瘤种选择”策略(以HER2突变NSCLC为突破口)与国际厂商传统集中HER2高表达肿瘤的策略形成明显区隔。随着瑞康曲妥珠单抗正式上市,该药有望成为国内HER2突变NSCLC患者的新选项,同时推动国产ADC技术在全球的竞争力提升。
04 总结与展望
自从发现HER2作为治疗靶点以来,基于HER2的治疗策略在乳腺癌以外的应用越来越广泛,这得益于分子肿瘤谱的临床应用增加和更有效的HER2靶向疗法的出现。在胃癌/胃食管交界处癌(G/GEJC)、结直肠癌(CRC)和非小细胞肺癌(NSCLC)中,HER2靶向治疗策略已成为FDA批准的标准护理方案,而在预后不良的胆道癌(BTCs)中,HER2靶向药物已显示出持久的反应和显著的临床效果。此外,强效的抗HER2 ADC药物T-DXd最近被FDA批准用于治疗HER2过表达的实体瘤,无论其组织学类型如何,定义为HER2 IHC 3+。
尽管已识别出对这些不同药物的各种潜在耐药机制,但已经开发出作用机制不同的药物来靶向HER2或其下游信号通路。随着我们对癌症发生过程和癌症生物学的理解不断加深,将开发新的治疗策略以克服潜在的耐药机制并提高现有HER2靶向药物的抗肿瘤效力。正在测试的HER2靶向治疗策略与各种药物联合使用,包括免疫疗法和DDR抑制剂,以协同改善临床结果。基于在乳腺癌和G/GEJC中HER2靶向疗法取得的进展,确定不同癌症类型中不同HER2靶向疗法的准确预测生物标志物将是关键。此外,扩展HER2靶向药物的适应症,现在包括HER2低表达癌症,将进一步推进HER2导向治疗策略的进展,最终增加能够从这种治疗中受益的患者数量。
★
临床3期抗体药物偶联物突破性疗法上市批准引进/卖出
▼
9月26-29日,BiG将召开以「创新出海 云起潮升」为主题的第十一届IMPACT年会,会上将举办大分子破界论坛,会上,百济神州副总裁、百济研究院院长严民宏博士将做首秀分享。
近年来,中国生物医药创新活力迸发,尤其在抗体药物偶联物(ADC)领域取得显著突破。在2025年世界肺癌大会(WCLC)上,中国企业的ADC临床数据惊艳国际舞台。
映恩生物、百利天恒和复宏汉霖作为国内创新药企的代表,凭借各自的技术平台和核心ADC产品,在激烈竞争的全球ADC赛道中脱颖而出。
作者|小鱼
要点速览:
● 映恩的平台化迭代、百利的双抗ADC技术、复宏汉霖的IO-ADC协同,代表了当前国内ADC创新的三大主流技术方向。
● 映恩生物的DB-1303在HER+乳腺癌中已达到III期研究主要终点。
● 百利天恒的iza-bren(BL-B01D1)在EGFR突变非小细胞肺癌(NSCLC)的治疗中显示了突破性的疗效(ORR高达100%),并已进入优先审评程序。
● 复宏汉霖的HLX43(PD-L1 ADC)因其独特的双重机制和在不限PD-L1表达人群中的疗效而备受关注,其国际多中心II期研究已启动。
表1 三家公司核心产品临床进展
01
映恩生物
双线布局,技术平台优势凸显
1.1 DITAC平台技术驱动
图1 四大ADC技术平台
映恩生物建立了四大ADC技术平台:DITAC(差异化免疫毒素ADC平台)、DIBAC(双特异性ADC平台)、DIMAC(双模态ADC平台)和DUPAC(双载荷ADC平台),这些平台构成了其管线拓展的技术基础。
图2 基于拓扑异构酶抑制剂的ADC平台
其中,DITAC平台采用拓扑异构酶I抑制剂作为载荷,通过优化的连接子技术,旨在降低脱靶毒性及增强抗肿瘤活性,包括旁观者杀伤效应。DB-1303(HER2 ADC)是基于该平台开发的,III期临床试验已达到主要终点,证明了该技术平台的成熟度。
1.2 稳步推进成熟靶点,积极布局新兴靶点
映恩生物采取了渐进式开发策略。一方面推进HER2这类成熟靶点的深度开发,另一方面布局ADAM9等新兴靶点。
图3映恩生物管线
① 稳步推进成熟靶点HER2、B7-H3
映恩生物的核心产品DB-1303(HER2 ADC)在HER2阳性不可切除或转移性乳腺癌的III期临床试验中,经独立数据监查委员会(IDMC)评估,已达到了由盲态独立中心阅片(BICR)评估的PFS主要研究终点。其合作伙伴BioNTech正计划于2025年提交该药用于HER2表达晚期子宫内膜癌的上市许可申请(BLA)。
这也是一项头对头罗氏HER2 ADC药物T-DM1的III期临床试验,但胜利数据还未公布。
图4 2025年美国临床肿瘤学会(ASCO)年会报告现场
另一款核心产品DB-1311(B7-H3 ADC)在治疗重度经治去势抵抗性前列腺癌(CRPC)的I/II期临床试验中显示了良好的初步疗效(52例):经确认的客观缓解率(cORR)为30.8%,疾病控制率(DCR)为90.4%。
②映恩生物积极布局新兴靶点ADAM9
公司开发的ADAM9 ADC药物DB-1317已悄然在美国临床试验官网登记I期研究,计划招募183例晚期实体瘤患者,试验地点涵盖美国和澳大利亚的多中心。
ADAM9是一个曾有项目折戟、全球仅5款在研的靶点。映恩生物选择这一靶点,体现了其差异化的研发策略。
02
百利天恒
双抗ADC惊艳WCLC,疗效数据创纪录
2.1 双抗ADC技术突破
百利天恒选择了更具挑战性的双特异性ADC技术路径,通过同时靶向两个相关受体,增强了肿瘤特异性结合能力,减少了脱靶毒性。
2.2 临床聚焦肺癌领域突破
图5 百利天恒管线
百利天恒选择了专注肺癌领域的策略,敏锐地抓住了肺癌治疗中未满足的临床需求,特别是EGFR-TKI耐药后患者的治疗困境。通过精准定位这一细分市场,百利天恒实现了快速突破。EGFR×HER3双抗ADC药物iza-bren(BL-B01D1)在2025年WCLC大会上备受关注。
①联合用药一线治疗
iza-bren联合奥希替尼一线治疗EGFR突变非小细胞肺癌(NSCLC)的II期研究取得突破性结果:在40例接受2.5mg/kg剂量治疗的患者中,客观缓解率(ORR)达到100%,12个月无进展生存率为92.1%,总生存率为94.8%,所有患者靶病灶均缩小,安全性可控。
②单药治疗后线治疗
iza-bren单药治疗局晚期或转移性EGFR突变非小细胞肺癌患者的I/II期研究同样取得重要进展。在171例总人群中(50例患者既往接受过EGFR-TKI治疗),中位PFS达12.5个月,ORR为66%,疾病控制率(DCR)为90%,18个月总生存(OS)率为69.2%,疗效覆盖不同EGFR突变亚型,安全性良好。
基于这些积极结果,其III期注册研究已在中国启动,并且该药已于2025年9月5日被国家药品监督管理局药品审评中心(CDE)纳入优先审评,拟用于治疗复发性或转移性鼻咽癌。
03
复宏汉霖
PD-L1 ADC崭露头角,高效低毒优势明显
3.1 IO-ADC协同创新
图6 复宏汉霖技术平台
复宏汉霖有Hinova TCE平台、Hanjugator™ ADC平台和HAI Club三大技术平台,开创了免疫治疗-ADC协同策略,其PD-L1 ADC药物具有毒素精准杀伤(ADC)与肿瘤免疫治疗(IO)双重作用机制。这种设计使药物不仅能直接杀伤肿瘤细胞,还能通过阻断PD-L1/PD-1通路激活T细胞免疫反应,产生“双刃剑”效应。
3.2 广谱抗肿瘤布局
图7 复宏汉霖管线
复宏汉霖采取了广谱开发策略。
①创新药HLX43:高效、低毒、带有I/O功能,已进入全球II期临床
创新PD-L1 ADC新药HLX43不仅在非小细胞肺癌中显示疗效,还在其他实体瘤中展现出治疗潜力。这种不依赖生物标志物筛选的开发策略,扩大了药物的适用人群,为后续商业化奠定了更广阔的基础。同时,公司还探索HLX43与其它药物的联合治疗方案,进一步拓展其应用场景。
图6 HLX43分子结构
HLX43在9月6日的WCLC大会上引起广泛关注。HLX43在治疗晚期/转移性实体瘤的临床I期更新数据中,显示出优异的抗肿瘤活性和良好的安全性。
图7 WCLC大会现场
HLX43在EGFR野生型非鳞状NSCLC患者中表现出色,客观缓解率达到46.7%,其中2.5mg/kg剂量组更达到60%。这意味着HLX43对全球最大癌种中占比最大的患者群体具有Best-in-class优势。
表2 肿瘤反应亚组分析
更值得关注的是,HLX43展示了不依赖生物标志物筛选的广谱性。NSCLC各个亚组均展现出优异疗效,在晚期的脑转移NSCLC患者中,仍带来显著的治疗获益,确认的客观缓解率为36.4%,疾病控制率达100%。
表3 安全性分析
在安全性方面,HLX43表现出血液毒性显著较低的优势。最常见的≥3级治疗相关不良事件为贫血(19.6%)、白细胞计数减少(19.6%)、中性粒细胞计数减少(16.1%)及淋巴细胞计数减少(12.5%),血小板计数减少仅3.6%。
②H药实现肺癌一线全覆盖,探索更广泛人群治疗潜力
表4 ASTRUM-002临床研究结果
此外,复宏汉霖的H药(斯鲁利单抗),一款抗PD-1单抗,其一线治疗晚期非鳞状非小细胞肺癌(nsqNSCLC)的III期临床研究(ASTRUM-002)结果也在WCLC上发布:H药联合化疗组(B组)的中位PFS达到11.0个月,较化疗组显著延长5.4个月,疾病进展风险降低45%;以及多项H药免疫联合疗法的IIT研究结果的简短口头报告,印证了H药在ES-SCLC更多亚组人群中的疗效。H药目前已在中国、英国、德国等近40个国家和地区获批上市。
小结
在ADC的星辰大海中,中国药企正从曾经的追随者,蜕变为全球创新的领航者。映恩、百利、复宏汉霖们已经证明,我们不仅能做出“me-too”,更能开创“me-better”,甚至定义“first-in-class”。
但同时全球竞争仍旧激烈,国际药企巨头也在积极布局ADC领域,如辉瑞旗下PD-L1 ADC药物PF-08046054(SGN-PDL1V)已正式启动III期临床试验;第一三共与默沙东合作的ADC药物I-DXd可能成为首个获批用于小细胞肺癌的ADC;强生正在探索ENPP3这一新靶点,布局了TCE双抗和ADC药物;礼来进入PTK7 ADC领域;罗氏在ADC领域达成了多项合作协议。
因此,唯有持续深耕技术壁垒、坚守临床价值、保持敬畏之心,方能持续领航。
参考资料
1.https://www.dualitybiologics.com/
2.http://www.baili-pharm.com/index.aspx
3.https://www.systimmune.com/news---systimmune-inc-asco-congress-2025
4.https://www.henlius.com/
5.https://www.asco.org/annual-meeting
近期活动
9月26-29日,BiG将召开以「创新出海 云起潮升」为主题的第十一届IMPACT年会,届时上海杭州双城联动,向全球化,再出发!
▼
时间及地点:
9月26-27日 上海-张江希尔顿;规模:600-800人
9月29日 杭州-西湖大学;规模:100人(需单独注册)
报名入口
9月26-27日 上海会议注册码
9月29日 西湖大学会议注册码
会议门票:审核制(免费),不含餐。优先Biotech/Pharma、临床、院校科学家、投资机构
付费门票:详见上二维码,注册内信息
1v1资本对接会
路演项目征集
可扫码填写项目信息
现场开放1v1咨询台,与各大头部VC面对面
▼
电脑端链接:https://event.mymova.com/spa4/#/?eventname=11bigvc
共建Biomedical创新生态圈!
如何加入BiG会员?
抗体药物偶联物临床3期优先审批ASCO会议临床2期
近日,BioBAY园内上市企业信达生物宣布其自主研发的重组抗胰岛素样生长因子1受体(IGF-1R)单抗药物信必敏®(替妥尤单抗N01注射液)正式获得中国澳门特别行政区药品监管部门批准上市,用于甲状腺眼病的治疗,该药物是澳门首个获批的中国自主研发IGF-1R单抗。
信必敏®此次成功在澳门获批上市,是继2025年3月获中国国家药品监督管理局(NMPA)批准后,在市场准入方面取得的又一重要进展。它不仅为澳门乃至亚太地区患者提供了更先进、更便捷的治疗选择,也体现了信达生物持续拓展全球创新药物可及性的企业承诺。
作为中国首个获批的IGF-1R抗体类药物
信必敏®通过精准靶向IGF-1R
并阻断下游信号通路
为中国甲状腺眼病治疗带来了突破性改变
信必敏®的上市,填补了国内该领域70年的治疗空白,彻底扭转了中重度活动期甲状腺眼病“无药可治”的困境,推动诊疗进入“靶向干预、高效缓解、无创治疗”的新阶段。
III期临床研究结果显示,信必敏®具有显著疗效和良好安全性。经24周治疗,患者突眼回退≥2 mm的应答率达到85.8%,83.5%的患者临床活动评分(CAS)降至0或1分,复视改善率达66%。同时,患者在疼痛缓解、眼睑闭合、外观恢复及生活质量量表(GO-QoL)评分等方面均表现出全面改善。
信达生物综合管线首席研发官钱镭博士表示:“信必敏®不仅是信达生物创新研发的重要成果,更是回应患者迫切需求的切实行动。我们非常高兴能够将这一创新疗法带给澳门患者,并将继续积极推进其在全球市场的注册进程。”
信达生物
信达生物于2011年在苏州工业园区成立,致力于研发、生产和销售肿瘤、自身免疫、代谢、眼科等重大疾病领域的创新药物,于2018年10月在港交所上市。企业已有16款产品获批上市,还有多个品种处于上市申请或临床研究阶段。公司已与礼来、罗氏、赛诺菲、Incyte和MD Anderson癌症中心等国际合作方达成30多项战略合作。
信达生物在不断自研创新药物、谋求自身发展的同时,秉承经济建设以人民为中心的发展思想。多年来,信达生物患者援助项目已惠及20余万普通患者,药物捐赠总价值36亿元人民币。
▌文章来源:苏州工业园区科技招商中心
责编:何文正
审核:任旭
推荐阅读
BioBAY园内188家!2025年第一批科技型中小企业名单出炉
上市企业丨映恩生物:首个3期临床成功!DB-1303达到了BICR评估的PFS主要终点
国内首款经血管主动脉瓣反流介入瓣膜获批上市!
100 项与 Trastuzumab Pamirtecan 相关的药物交易