预约演示
更新于:2025-10-30
ESG-401
更新于:2025-10-30
概要
基本信息
药物类型 ADC |
别名 Humanized anti-Trop2-SN-38 antibody conjugate、Humanized mAb anti-TROP-2 program、重组人源化抗Trop2单抗-SN38偶联物 (诗健生物) + [6] |
作用方式 抑制剂 |
作用机制 TOP1抑制剂(DNA拓扑异构酶I抑制剂)、Trop-2抑制剂(肿瘤相关钙信号传感器2抑制剂) |
在研适应症 |
在研机构 |
最高研发阶段临床3期 |
首次获批日期- |
最高研发阶段(中国)临床3期 |
特殊审评突破性疗法 (中国) |
登录后查看时间轴
结构/序列
分子式C22H20N2O5 |
InChIKeyFJHBVJOVLFPMQE-QFIPXVFZSA-N |
CAS号86639-52-3 |
使用我们的ADC技术数据为新药研发加速。
登录
或
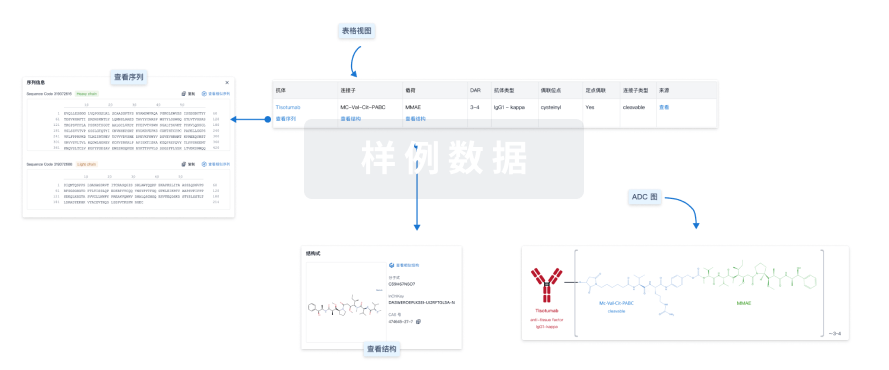
Sequence Code 27958L

当前序列信息引自: *****
Sequence Code 319372635H

当前序列信息引自: *****
关联
4
项与 ESG-401 相关的临床试验NCT06732323
A Randomized, Open-label, Phase III Study of ESG401 Versus Investigator's Choice Chemotherapy as First-line Treatment in Patients With Unresectable Recurrent or Metastatic Triple-Negative Breast Cancer
The aim of this study is to evaluate the efficacy and safety of ESG401 as first-line treatment in patients with unresectable recurrent or metastatic triple-negative breast cancer.
开始日期2025-09-04 |
申办/合作机构 |
NCT06383767
A Open-label, Randomized, Multicenter Phase III Study of ESG401 Versus Investigator's Choice Chemotherapy in Patients With Locally Advanced or Metastatic HR+/HER2- Breast Cancer Who Had Failed at Least One Line of Chemotherapy
The aim of this study is to evaluate the efficacy and safety of ESG401 in patients with unresectable locally advanced or metastatic HR+/HER2- breast cancer.
开始日期2024-07-11 |
申办/合作机构 |
NCT05060276
A Phase 1B, Dose-Escalation Study of the Safety and Preliminary Efficacy of an Anti-Trop2 Antibody Drug Conjugate (STI-3258) in Patients With Relapsed or Refractory Solid Tumors
This is a phase 1b, open-label, dose-escalation study o STI-3258 administered intravenously in subjects with relapsed or refractory solid tumors.
开始日期2022-12-01 |
申办/合作机构 |
100 项与 ESG-401 相关的临床结果
登录后查看更多信息
100 项与 ESG-401 相关的转化医学
登录后查看更多信息
100 项与 ESG-401 相关的专利(医药)
登录后查看更多信息
1,843
项与 ESG-401 相关的文献(医药)2025-08-01·TOXICOLOGY
High-content screening morphological analysis to evaluate hepatic apoptosis induced by plant alkaloids in a Chang cell model
Article
作者: Choi, Hwa Young ; Jang, Sihyeon ; Park, Young Eun ; Chun, Hyang Sook ; Park, Su Been
As interest in plant-derived compounds and their application in the pharmaceutical and functional food industries has increased, the rapid detection of chemical toxicity has become increasingly important for developing safe products. High-content screening (HCS) can quantify cellular and organelle morphological changes through image analysis; however, most HCS studies on apoptosis, a key toxicological event, have focused on the expression of apoptosis-related genes or proteins. In this study, we aimed to verify whether apoptosis can be detected solely based on cellular morphological changes. Chang cells were treated with staurosporine (STS), a well-known apoptosis inducer, and the morphological changes in the cells were quantified using HCS assays. The correlation between these HCS morphological descriptors and apoptosis rates measured using flow cytometry was determined. Chang cells were also treated with several plant-derived alkaloids known to induce apoptosis, and the same process was performed. The correlation coefficients, which were used to evaluate the correlation between HCS descriptors and apoptosis rates after STS treatment, ranged from 0.64 to 0.98, with 13 descriptors showing significant correlations. In contrast, the highest correlation coefficients between HCS descriptors and apoptosis rates after treatment with 1 of the 12 alkaloids investigated were determined to be 0.75 (at 10 μg/ml) and 0.49 (at 100 μg/ml). The apoptosis-related morphological changes induced by STS and alkaloids were observed using confocal microscopy. The present study demonstrates that HCS assays can detect apoptosis solely based on cellular morphological changes, providing a potential tool for rapid toxicity screening in early product development.
2025-06-01·BIOORGANIC CHEMISTRY
Redox dyshomeostasis-driven prodrug strategy for enhancing camptothecin-based chemotherapy: Selenization of SN38 as a case study
Article
作者: Liu, Yu ; Zhao, Jintao ; Fang, Jianguo ; Zhang, Baoxin ; Xi, Junmin ; Zhang, Linjie
Harnessing the modulation of redox homeostasis represents a promising anticancer strategy. Here, we design and evaluate Se-SN38, a prodrug of the camptothecin (CPT) derivative 7-ethyl-10-hydroxycamptothecin (SN38) with a cyclic five-membered diselenide moiety for redox-triggered activation. We demonstrate that Se-SN38 exhibits superior cytotoxicity in various cancer cell lines over the parent drug SN38 or the control prodrug S-SN38, a sulfur analogue of Se-SN38. This increased potency is attributed to the efficient release of SN38 and induction of oxidative stress, as demonstrated by a significant rise in reactive oxygen species production, along with a marked depletion of cellular total thiols and a decreased GSH/GSSG ratio. Furthermore, Se-SN38 treatment leads to inhibition of thioredoxin reductase activity, disruption of mitochondrial membrane potential, and induction of DNA damage, culminating in apoptosis. These findings suggest that Se-SN38 represents a promising strategy to enhance the therapeutic efficacy of CPT derivatives by exploiting the unique redox-active properties of cyclic five-membered diselenide to induce oxidative stress and apoptosis.
2025-06-01·JOURNAL OF PHARMACEUTICAL AND BIOMEDICAL ANALYSIS
Development and optimization of a high-throughput LC-MS/MS method for the simultaneous determination of Exatecan and its Cathepsin B-sensitive prodrug, and ARV-825 in rat plasma: Application to pharmacokinetic study
Article
作者: Li, Jing ; Zhang, Tianhong ; Ran, Xiaohua ; Jiang, Qikun ; Liu, Yangyang ; Yan, Qing ; Zhang, Jiaming ; Xu, Xiaolan ; Bai, Chenxia
For most cancers, the combination of chemotherapy drugs is a promising approach. The combination of DNA damage agent Exatecan and proteolysis targeting chimera (PROTAC) agent ARV-825, which is a selective bromodomain-containing protein 4 degrader, can further improve efficacy through the DNA damage-repair mechanism. The Cathepsin B-sensitive prodrug with high albumin affinity of Exatecan (C14-VC-PAB-Exa) was introduced and co-encapsulated with ARV-825 into the nano-drug delivery system for improving the physicochemical properties of the two drugs. To promote the translation of Exatecan and the PROTAC into the clinics, it is important to develop a reliable and high-throughput bioanalytical method for the simultaneous determination of Exatecan, C14-VC-PAB-Exa, and ARV-825 that can evaluate the pharmacokinetic behaviors of the analytes. In this study, an HPLC-MS/MS method after preparation by one-step protein precipitation was developed and fully validated. The analytes were eluted completely on a ZORBAX SB-C18 column by gradient elution. Multiple reaction monitoring mode with positive electrospray ionization was applied to quantify the analytes. The validated method on selectivity, linearity (r ≥ 0.995), precision and accuracy (< 15 %), extraction recovery (> 88.0 %), matrix effect (< 9.1 %), carry-over, and stability were within the predefined acceptance criteria. The method was successfully applied to the pharmacokinetic study of Exatecan, C14-VC-PAB-Exa, and ARV-825 in rats for the first time. The proposed robust and economical method will be an alternative bioanalytical procedure for Exatecan and ARV-825 in the future. What is more, the present work could provide a reference for the clinical combination of the two drugs.
56
项与 ESG-401 相关的新闻(医药)2025-10-25
以下内容由IdeaFromAI-Plus版工具总结:
教程:¥1.99元起让文献整理效率翻倍!——导师追问进度条,你已经优雅交出word终稿。
肿瘤相关钙信号转导蛋白2(TROP2),又称TACSTD2,是一种在多种实体瘤中高表达的跨膜糖蛋白,其过表达通常与肿瘤的侵袭性、转移能力增强以及患者预后不良密切相关。这些特性使TROP2成为肿瘤靶向治疗,尤其是抗体药物偶联物(ADC)开发的理想靶点。ADC通过将高特异性的单克隆抗体与强效的细胞毒性药物(载荷)通过连接子偶联,实现了对肿瘤细胞的精准打击,同时降低了对正常组织的损伤。近年来,以Sacituzumab govitecan (SG)和Datopotamab deruxtecan (Dato-DXd)为代表的TROP2靶向ADC在多种实体瘤,特别是三阴性乳腺癌(TNBC)和非小细胞肺癌(NSCLC)的治疗中取得了突破性进展,改变了临床实践格局。本综述旨在系统性地梳理和论述TROP2的生物学功能、其在不同癌种中的表达谱与临床意义、TROP2-ADC的设计原理与作用机制、主要在研及已上市药物的临床前与临床数据,并深入探讨TROP2-ADC治疗所面临的挑战,如耐药性机制和毒副作用管理。通过整合现有研究,本文旨在全面展现TROP2靶向ADC作为一类革命性抗肿瘤药物的科学基础、临床价值及当前局限性。关键词
TROP2;抗体药物偶联物;Sacituzumab govitecan;Datopotamab deruxtecan;肿瘤治疗;耐药性;间质性肺病
引言
抗体药物偶联物(ADC)作为一种新兴的靶向治疗策略,被誉为“生物导弹”,它巧妙地结合了单克隆抗体的高度靶向性与小分子化疗药物的强大杀伤力,旨在实现对癌细胞的精准杀灭,并最大限度地减少全身性毒副作用。一个典型的ADC由三个核心部分组成:特异性识别肿瘤表面抗原的抗体、通过化学连接子(Linker)与抗体相连的细胞毒性载荷(Payload),以及发挥最终杀伤作用的载荷。理想的ADC靶点应在肿瘤组织中高度且均一地表达,而在正常组织中表达量极低或不表达,从而为药物提供一个足够宽的治疗窗口。
在众多潜在的ADC靶点中,肿瘤相关钙信号转导蛋白2(TROP2, Trophoblast cell-surface antigen 2),其基因名为TACSTD2,近年来受到了前所未有的关注。TROP2是一种I型跨膜糖蛋白,最初在滋养层细胞中被发现,后续研究证实其在多种上皮来源的恶性肿瘤中广泛过表达,包括乳腺癌、肺癌、胃癌、结直肠癌、胰腺癌、膀胱癌、宫颈癌等[1, 2]。TROP2不仅是一个细胞表面标志物,更深度地参与了肿瘤的多种病理生理过程。研究表明,TROP2的过表达能够通过激活PI3K/Akt、NF-κB/IL-8等信号通路,显著促进肿瘤细胞的增殖、侵袭、迁移和上皮间质转化(EMT),并抑制细胞凋亡[3, 4, 5]。因此,TROP2的高表达水平常常与肿瘤的侵袭性表型、更晚的临床分期以及患者更差的生存预后紧密相连[6, 7, 8, 9]。
基于TROP2在肿瘤生物学中的重要作用及其理想的靶点特性,针对TROP2的ADC药物开发已成为当前肿瘤药物研究的热点领域。以美国FDA批准上市的Sacituzumab govitecan (SG)为代表的TROP2-ADC,已在临床上证明了其在治疗难治性、转移性三阴性乳腺癌(TNBC)和尿路上皮癌中的卓越疗效,并正在向更多癌种拓展[10]。与此同时,Datopotamab deruxtecan (Dato-DXd)等一系列新型TROP2-ADC也展现出强大的抗肿瘤活性和临床潜力。本综述将依据现有已发表的研究成果,详细阐述TROP2的生物学特性及其作为治疗靶点的价值,深入剖析TROP2-ADC的分子设计、多样化的作用机制,系统总结主要TROP2-ADC药物在不同实体瘤中的研究进展,并对治疗过程中出现的毒副作用管理和耐药性等关键挑战进行全面论述。正文1. TROP2的生物学功能及其在肿瘤治疗中的靶点价值1.1 TROP2的分子结构、信号调控与生物学功能
TROP2是一种分子量约为40kDa的单次跨膜糖蛋白,其结构包括一个胞外结构域(ECD)、一个跨膜区和一个短的胞内尾区。胞外结构域是抗体识别和结合的主要区域,而胞内尾区虽然短小且无已知的酶活性,但包含一个磷脂酰肌醇4,5-二磷酸(PIP2)结合位点,这对于其介导下游信号至关重要。TROP2的生物学功能复杂多样,在胚胎发育过程中,它参与调控器官形成,例如在胎儿肺部细胞增殖中发挥作用[11]。然而,在肿瘤细胞中,TROP2的功能被“劫持”,成为驱动恶性进展的关键分子。
多项研究揭示了TROP2参与调控的关键信号通路。在口腔癌中,TROP2被证实可以通过激活PI3K/Akt信号通路来促进肿瘤的转移[3]。在三阴性乳腺癌(TNBC)中,一种名为Uc003xsl.1的长链非编码RNA能够激活NF-κB/IL-8信号轴,进而促进TNBC的进展,而TROP2在此过程中扮演了重要角色[4]。此外,在皮肤鳞状细胞癌中,TROP2同样被发现能够促进细胞的增殖和迁移[5]。更有研究发现,TROP2能够通过抑制p16蛋白的表达来促进口腔癌细胞内的钙离子释放,这可能是一种新的促癌机制[12]。在非小细胞肺癌(NSCLC)的吉非替尼耐药模型中,TROP2被发现能与胰岛素样生长因子2受体(IGF2R)结合,从而诱导对吉非替尼的耐药性,这与上皮间质转化(EMT)过程中的Vimentin上调和E-cadherin下调有关[13, 14]。这些研究共同描绘了TROP2作为一个多效性的信号节点,通过多种途径驱动肿瘤的生长、存活、转移和耐药,从而确立了其作为治疗靶点的坚实生物学基础。1.2 TROP2在多种实体瘤中的广泛表达与临床预后意义
TROP2作为ADC靶点的另一个关键优势在于其在多种上皮源性实体瘤中的广泛高表达,这为TROP2-ADC的“泛癌种”应用潜力提供了依据。
乳腺癌:TROP2在乳腺癌中,尤其是在预后最差、治疗选择有限的三阴性乳腺癌(TNBC)亚型中,呈现出高水平表达。研究显示,高达85%的高级别乳腺癌病例为TROP2阳性[15]。TROP2的高表达与TNBC的不良预后显著相关,使其成为该亚型极具吸引力的治疗靶点[7, 16]。TROP2靶向ADC,如SG和Dato-DXd,已在TNBC治疗中显示出革命性的疗效,甚至在脑转移这种棘手的临床场景中也观察到了活性[10, 17]。
肺癌:在非小细胞肺癌(NSCLC)中,TROP2的过表达同样普遍,并且是预测患者不良预后的独立因素[8, 18]。一项研究发现,TROP2的高表达与NSCLC患者接受免疫检查点抑制剂治疗的预后不良相关,提示TROP2可能参与了免疫抑制微环境的形成[19]。另一项研究则观察到TROP2与PD-L1的表达在肺癌中可能存在负相关关系,这为TROP2-ADC与免疫疗法的联合应用提供了复杂的背景和潜在的理论依据[20]。值得注意的是,TACSTD2基因的上调也被发现是肺部感染过程中的一种早期反应,这提示其在炎症和组织修复中也可能扮演一定角色[21]。
消化系统肿瘤:TROP2在消化系统肿瘤中也扮演着重要角色。在胃癌中,TROP2的高表达与TNBC类似,同样普遍存在[16]。在结直肠癌(CRC)中,TROP2的表达与肿瘤的侵袭性表型相关,且其高表达与转移性结直肠癌(mCRC)患者的预后不佳有关[22, 23]。针对胃癌,有研究利用TROP2抗体修饰的黑磷纳米片来增强光热治疗的效果,展示了靶向TROP2策略的多样性[24]。在胰腺癌中,TROP2同样高表达,使其成为ADC的有效靶点[25, 26]。在胆管癌(CCA)中,TROP2的表达也相当广泛,研究显示,使用SG能够有效抑制源自患者的胆管癌类器官(PDO)的生长,为TROP2-ADC治疗CCA提供了临床前证据[27, 28]。一项针对多种消化系统癌症的分析指出,TROP2的高表达与胃肠道印戒细胞癌(SRCC)、血管侵犯(VI)和神经侵犯(PNI)等恶性临床病理特征显著相关,进一步凸显了其作为预后不良标志物和治疗靶点的价值[29]。
妇科肿瘤:在妇科恶性肿瘤领域,TROP2的表达也为ADC治疗开辟了新途径。宫颈癌组织中普遍高表达TROP2、CEACAM5和CD138等ADC靶点,为开发针对这些靶点的ADC提供了理论基础[30]。在卵巢癌中,尽管不同ADC靶点的表达存在显著差异,但TROP2仍是一个重要的潜在靶点[31]。SG已被证明能够有效抑制卵巢癌的生长[32],而Dato-DXd也在TROP2阳性的卵巢癌患者中显示出临床活性[33]。此外,在子宫内膜癌中,TROP2是ADC药物开发的重要靶点之一[34, 35, 36],SG在罕见的子宫浆液性癌中也显示出靶向TROP2的潜力[37]。外阴鳞状细胞癌(VSCC)中也普遍表达TROP2,且其表达水平与特定的分子亚型相关[38, 39]。
泌尿系统肿瘤及其他:在膀胱癌中,TROP2和NECTIN4(另一种重要的ADC靶点)均呈现高表达,为单药或联合靶向治疗提供了机会[40]。TROP2在膀胱癌的各个亚型中均有广泛表达,并且其表达与EpCAM相关[41, 42]。在乳腺外佩吉特病(EMPD)中,TROP2的高表达同样与不良预后相关,提示TROP2-ADC可能成为一种新的治疗选择[43, 44]。
综上所述,TROP2在众多实体瘤中的高表达率及其与不良预后的强关联性,共同奠定了其作为广谱抗肿瘤ADC靶点的坚实基础,驱动了大量靶向TROP2的药物研发活动。2. TROP2靶向ADC的设计原理与抗肿瘤作用机制
TROP2-ADC的成功依赖于其各个组成部分的精心设计,包括抗体、连接子和载荷,以及它们协同作用所产生的独特抗肿瘤机制。2.1 TROP2-ADC的核心组成与关键技术
抗体部分:ADC的“导航系统”是其抗体部分。目前开发的TROP2-ADC主要采用人源化单克隆抗体,如SG和Dato-DXd所使用的抗体,以降低免疫原性并延长半衰期。为了进一步优化药物特性,研究人员正在探索多种新型抗体形式。例如,开发新型人源化抗TROP2抗体IMB1636 [45],以及基于此抗体构建的ADC药物hIMB1636-MMAE [46]。纳米抗体(Nanobody)由于其分子量小、组织穿透性强、生产成本低等优点,也成为TROP2靶向治疗的新选择。已有研究开发了靶向TROP2的纳米抗体药物偶联物,并在胰腺癌模型中显示出抗肿瘤活性[26]。此外,双特异性抗体,如同时靶向TROP2和T细胞表面CD3分子的F7AK3,能够招募T细胞直接杀伤肿瘤细胞,代表了一种不同的免疫治疗策略[47]。
连接子与偶联技术:连接子的设计对于ADC的成败至关重要。它必须在血液循环中保持稳定,以防止载荷过早释放导致全身毒性;同时,在进入靶细胞后,又能被特异性地切断以释放活性载荷。目前TROP2-ADC多采用可裂解连接子,如SG中的CL2A连接子和Dato-DXd中的四肽连接子,它们可以在肿瘤细胞内的酸性环境或特异性酶(如组织蛋白酶B)作用下断裂。此外,药物-抗体比(DAR)和偶联位点也显著影响ADC的均一性、稳定性及药代动力学。传统的随机偶联技术产生的ADC混合物不均一,而定点特异性偶联技术能够生产出DAR均一的ADC。例如,新型定点特异性TROP2 ADC gsADC 3b就显示出良好的稳定性[48]。
载荷/弹头:载荷是ADC的“弹头”,其效力直接决定了ADC的杀伤能力。针对TROP2的ADC主要采用了两类高效载荷:
·拓扑异构酶I(TOP1)抑制剂:这是目前最成功的TROP2-ADC载荷类型。Sacituzumab govitecan (SG)的载荷是SN-38,是化疗药伊立替康的活性代谢物。Datopotamab deruxtecan (Dato-DXd)的载荷是Deruxtecan (DXd),一种新型、高效的TOP1抑制剂。TOP1抑制剂通过嵌入DNA并稳定TOP1-DNA复合物,导致DNA单链和双链断裂,激活DNA损伤应答(DDR),最终诱导细胞周期G2/M期阻滞、复制应激和细胞凋亡[49]。DXd的效力据称是SN-38的数倍,并且具有较高的细胞膜渗透性,这为其强大的旁观者效应奠定了基础[50]。
·其他类型的载荷:为了应对耐药性和拓宽应用,研究人员也在探索其他类型的载荷。微管蛋白抑制剂MMAE(单甲基澳瑞他汀E)被用于构建hIMB1636-MMAE [46]。一种极具潜力的新型载荷是α-鹅膏蕈碱(Amanitin),它是一种RNA聚合酶II抑制剂,其作用机制与DNA损伤药物完全不同,可能对传统化疗耐药的肿瘤有效。一款靶向TROP2的新型Amanitin ADC在胰腺癌模型中就显示出高效低毒的特性[25]。2.2 TROP2-ADC的多重抗肿瘤作用机制
TROP2-ADC的抗肿瘤活性并非单一机制的结果,而是多种机制协同作用的体现。
直接细胞毒性作用:这是ADC最基本的作用机制。ADC通过抗体部分与肿瘤细胞表面的TROP2结合,随后整个复合物通过内吞作用被细胞吞入。在细胞内,ADC被转运至溶酶体。有研究指出,改善ADC的溶酶体运输效率可以显著提高其效力[51]。在溶酶体中,连接子被降解,释放出高浓度的细胞毒性载荷。载荷随后作用于其靶点(如DNA或微管),最终导致肿瘤细胞死亡。
旁观者效应(Bystander Effect):肿瘤组织往往具有高度异质性,并非所有癌细胞都表达TROP2。旁观者效应是指当ADC释放的载荷具有细胞膜渗透性时,它可以从TROP2阳性的靶细胞中扩散出来,进入并杀死邻近的TROP2阴性或低表达的肿瘤细胞。这一效应极大地扩展了ADC的杀伤范围,对于治疗异质性肿瘤至关重要。Dato-DXd的载荷DXd就具有很强的膜渗透性,因此表现出显著的旁观者效应。一项研究在TROP2过表达的癌肉瘤模型中证实了Dato-DXd的旁观者效应[52]。
免疫原性细胞死亡(Immunogenic Cell Death, ICD):传统的化疗药物通常诱导耐受性的细胞死亡,而某些药物,包括一些ADC载荷,可以诱导ICD。ICD的特征是垂死的癌细胞会释放一系列损伤相关分子模式(DAMPs),如钙网蛋白(CRT)的暴露、ATP的释放和HMGB1的释放。这些信号能够吸引并激活树突状细胞(DC),从而启动适应性抗肿瘤免疫反应。最近的研究表明,Datopotamab deruxtecan能够诱导具有ICD特征的细胞死亡,这提示TROP2-ADC不仅能直接杀伤肿瘤,还可能通过调动宿主免疫系统来协同抗肿瘤,为ADC与免疫疗法的联合提供了理论基础[53]。
其他创新机制:除了传统的ADC模式,研究人员还在探索基于TROP2靶向的其他治疗策略。例如,将TROP2靶向抗体与放射性核素(如177Lu)偶联,形成放射性免疫偶联物(RIC)。这种177Lu标记的ADC能同时通过靶向放射治疗和ADC的细胞毒性双重机制杀伤肿瘤,在实体瘤模型中显示出优于传统RIC的潜力[54]。3. 主要TROP2-ADC药物的临床前与临床研究进展
基于对TROP2靶点的深入理解,一系列TROP2-ADC药物被开发出来,并在临床试验中取得了令人瞩目的成果。3.1 Sacituzumab Govitecan (SG, Trodelvy®)
SG是首个获批上市的TROP2-ADC,由人源化抗TROP2抗体、可裂解的CL2A连接子和拓扑异构酶I抑制剂SN-38组成,DAR约为7.6。
在乳腺癌中的应用:SG的成功首先在转移性三阴性乳腺癌(TNBC)中得到验证。基于ASCENT研究的阳性结果,SG获批用于治疗既往已接受至少两种系统治疗(其中至少一种用于转移性疾病)的不可切除的局部晚期或转移性TNBC患者。其疗效显著优于标准化疗。此外,SG在新辅助治疗领域也显示出潜力,一项研究评估了其用于TNBC新辅助治疗的有效性[55]。
在其他癌种中的拓展:SG的适应症正在不断扩大。在小细胞肺癌(SCLC)中,SG与PD-1抑制剂帕博利珠单抗(pembrolizumab)的联合治疗显示出改善患者生存的希望[56]。在食管腺癌中,TROP2的表达水平被发现可以预测SG的治疗疗效[57]。此外,SG在治疗TROP2阳性的子宫浆液性癌和卵巢癌中也显示出抗肿瘤活性[37, 32]。这些数据表明,SG作为一种广谱抗癌药物具有巨大潜力,其应用范围远不止于乳腺癌和尿路上皮癌[58]。3.2 Datopotamab Deruxtecan (Dato-DXd, DS-1062)
Dato-DXd是另一款备受瞩目的TROP2-ADC,它由人源化抗TROP2抗体、一种稳定的可被肿瘤特异性蛋白酶切割的四肽连接子和高效的TOP1抑制剂Deruxtecan (DXd)组成,DAR约为4。其独特的设计赋予了它高稳定性和强大的旁观者效应。
在非小细胞肺癌(NSCLC)中的突破:Dato-DXd在NSCLC治疗领域取得了重大进展。TROPION-Lung01研究表明,对于经治的晚期NSCLC患者,Dato-DXd相比于多西他赛能够显著改善无进展生存期(PFS),尤其是在非鳞癌患者中获益更为明显[59]。基于这些数据,Dato-DXd有望成为NSCLC后线治疗的新标准[60]。药代动力学(PK)模型研究为Dato-DXd的剂量选择提供了指导,推荐的临床剂量为6 mg/kg [61]。
在乳腺癌及其他实体瘤中的潜力:Dato-DXd在HER2阴性乳腺癌,包括脑转移患者中也显示出令人鼓舞的抗肿瘤活性[62]。其活性被证实依赖于肿瘤细胞的TROP2表达水平[63]。此外,Dato-DXd在TROP2阳性的晚期宫颈癌、卵巢癌以及罕见的癌肉瘤中均观察到了临床疗效,进一步证实了其广谱抗癌的潜力[64, 33, 52]。一款名为ESG401的ADC,其结构与Dato-DXd相同,也在多项实体瘤中显示出疗效[65]。3.3 新型及在研的TROP2靶向疗法
除了SG和Dato-DXd,众多新型TROP2-ADC及其他靶向策略也正在积极开发中,旨在改善疗效、克服耐药或降低毒性。
·OBI-992:这是一款新型TROP2-ADC,临床前研究表明其在多种癌症模型中具有强大的抗肿瘤活性[66, 67]。更有头对头比较研究提示,OBI-992在某些模型中的表现可能优于Dato-DXd,显示出成为“best-in-class”药物的潜力[68]。
·SKB264 (MK-2870):这是一款由科伦博泰开发的TROP2-ADC,其载荷为一种新型的拓扑异构酶I抑制剂。临床前数据显示其活性优于IMMU-132(SG的早期代号)[69],目前正在全球范围内进行多项临床试验。
·其他ADC:如前所述,hIMB1636-MMAE(载荷为MMAE)和靶向TROP2的Amanitin ADC等,通过采用不同的载荷来探索新的抗癌机制和克服耐药的可能性[46, 25]。
·非ADC策略:除了ADC,靶向TROP2的CAR-T细胞疗法也成为研究热点。TROP2 CAR-T细胞在临床前模型中显示出强大的杀伤活性,并被认为是克服ADC耐药的一种潜在策略[70, 71]。然而,其在TNBC等实体瘤中的应用也伴随着潜在的脱靶毒性风险,需要进一步优化[72]。此外,靶向TROP2的适配体-药物偶联物、免疫毒素(Fab-PE24)以及用于肿瘤成像的分子探针和单域抗体等也都在积极研发中,展现了TROP2靶点开发的多样化前景[73,74, 75, 76]。4. TROP2-ADC治疗的挑战与应对策略
尽管TROP2-ADC取得了巨大成功,但其在临床应用中仍面临着毒副作用管理和耐药性两大核心挑战。4.1 毒副作用及其管理
ADC的治疗窗口虽然相比传统化疗有所拓宽,但并非没有毒性。TROP2-ADC相关的常见不良事件(AEs)主要包括血液学毒性、胃肠道反应和一些特殊的“类效应”毒性。
·间质性肺病/肺炎(ILD/Pneumonitis):这是与Deruxtecan系列ADC(包括T-DXd和Dato-DXd)相关的一个特别值得关注的严重不良事件。尽管发生率不高,但可能危及生命。一项关键研究揭示了其潜在机制:ADC可能通过Fcγ受体被肺泡巨噬细胞非特异性摄取,随后在肺部释放细胞毒性载荷,引发炎症反应和组织损伤[77]。因此,对接受Dato-DXd治疗的患者进行严密监测,早期识别并积极干预ILD/肺炎至关重要,这已成为临床实践中的标准操作规程[78]。
·血液学与胃肠道毒性:以SG为代表的ADC,其常见毒性主要包括中性粒细胞减少、贫血等血液学毒性,以及恶心、呕吐、腹泻等胃肠道反应。这些毒性通常是可预测且可管理的,通过支持性治疗(如使用粒细胞集落刺激因子G-CSF)和剂量调整,大部分患者可以耐受治疗[79]。4.2 耐药性机制与克服策略
随着TROP2-ADC的广泛应用,获得性耐药已成为一个不可避免的临床难题。其机制复杂多样,主要可分为靶点相关耐药和载荷相关耐药。
·耐药机制:研究发现,SG的耐药可能与TROP2(TACSTD2)基因的表达下调或功能丧失性突变有关,导致药物无法有效结合靶细胞。此外,作为载荷SN-38和DXd作用靶点的TOP1基因发生突变,也可能导致药物无法有效抑制其功能,从而产生耐药[80]。其他机制,如药物外排泵(如ABCG2)的上调、溶酶体功能障碍、细胞凋亡通路缺陷等,也可能参与耐药的形成。例如,COX-2的上调被发现与TROP2相关,并能促进结肠癌的耐药[81]。
·克服策略:
1.联合用药:联合治疗是克服耐药、增敏增效的重要策略。由于TOP1抑制剂主要通过制造DNA单链断裂发挥作用,这会激活DNA损伤应答(DDR)通路。因此,TROP2-ADC与DDR通路抑制剂的联合具有很强的协同作用。例如,与PARP抑制剂(PARPi)联合,可以形成“合成致死”效应,显著增强抗肿瘤活性[82]。同样,与ATR抑制剂联合也能产生协同作用[83]。
2.表观遗传调控:通过表观遗传学手段上调靶点表达是另一种有前景的策略。研究表明,使用表观遗传药物可以上调TROP2以及与药物敏感性相关的基因SLFN11的表达,从而增强SG的治疗效果[84]。
3.开发新疗法:当患者对一种TROP2-ADC耐药时,更换为另一种具有不同作用机制或载荷的药物可能有效。例如,对于SG耐药的肿瘤,或许可以尝试使用基于Amanitin的ADC。PLX038作为一种新型药物,也被提出可能对SG耐药的肿瘤有效[85]。此外,如前所述,开发TROP2 CAR-T细胞疗法是绕过ADC内化和载荷递送障碍的根本性策略,有望为ADC耐药患者提供新的治疗希望[70]。抑制自噬通路也被发现在胰腺癌中能增强TROP2-ADC的疗效,提示调控细胞自噬可能是克服耐药的新方向[86]。4.3 诊断与生物标志物
精准的患者选择是实现ADC个体化治疗的关键。
·TROP2表达检测:目前,免疫组化(IHC)是临床上评估TROP2表达水平的标准方法。然而,不同的抗体克隆、染色方案和判读标准可能导致结果存在较大差异,给患者分层带来挑战[87]。开发标准化的检测方法和可靠的抗体至关重要,例如TrMab-6就是一种被用于检测TROP2表达的单克隆抗体[88, 89]。定量检测HER2和TROP2双靶点表达的方法也正在开发中,以指导更复杂的治疗决策[90]。
·液体活检:循环肿瘤DNA(ctDNA)作为一种非侵入性的检测手段,在监测肿瘤动态变化和预测治疗反应方面显示出巨大潜力。一项研究表明,通过DNADX ctDNA测试监测TROP2等基因的改变,可以与接受SG治疗的乳腺癌患者的生存结局相关联,提示ctDNA有望成为指导TROP2-ADC治疗的动态生物标志物[91]。总结
综上所述,肿瘤相关钙信号转导蛋白2(TROP2)凭借其在多种实体瘤中的广泛高表达以及在驱动肿瘤恶性进展中的关键作用,已成为抗体药物偶联物(ADC)领域一个极其成功和充满希望的靶点。从生物学基础来看,TROP2通过调控PI3K/Akt、NF-κB等多个核心信号通路,深度参与肿瘤细胞的增殖、侵袭和转移,其高表达水平普遍预示着更差的临床预后,这为靶向治疗提供了坚实的理论依据。
TROP2-ADC的设计和开发体现了现代药物工程的精髓。通过优化抗体、连接子和载荷,研究人员成功地将高效的细胞毒性药物(如拓扑异构酶I抑制剂SN-38和Deruxtecan)精准递送至肿瘤部位。这些药物不仅通过直接杀伤TROP2阳性细胞发挥作用,更借助强大的旁观者效应清除邻近的异质性肿瘤细胞,并通过诱导免疫原性细胞死亡来潜在地激活宿主的抗肿瘤免疫,形成了多维度的抗癌机制。
以Sacituzumab govitecan和Datopotamab deruxtecan为代表的TROP2-ADC,已经在包括三阴性乳腺癌、非小细胞肺癌、泌尿系统肿瘤在内的多个癌种中取得了突破性的临床成功,显著改善了难治性、晚期患者的生存结局,并正在向更多的实体瘤适应症中拓展。与此同时,一系列具有不同载荷、不同抗体形式或创新偶联技术的新型TROP2-ADC和TROP2靶向疗法(如CAR-T)正处在积极的研发管线中,预示着该领域的持续繁荣。
然而,TROP2-ADC的临床应用也伴随着明确的挑战。以间质性肺病为代表的严重毒副作用要求临床医生具备高度的警惕性和专业的管理能力。同时,获得性耐药机制的阐明,如靶点丢失和载荷靶点突变,以及开发相应的克服策略,如合理的联合用药和序贯治疗,是决定TROP2-ADC长期临床价值的关键。精准的生物标志物,包括标准化的TROP2表达检测和动态的液体活检监测,对于实现患者的精准筛选和个体化治疗至关重要。
总而言之,TROP2靶向ADC的崛起是精准肿瘤学发展历程中的一个里程碑。它不仅为众多束手无策的晚期癌症患者带来了新的生命曙光,也深刻地改变了多个癌种的治疗范式。尽管挑战依然存在,但基于现有坚实的科学证据和临床数据,TROP2-ADC已经确立了其作为现代抗癌药物库中基石之一的重要地位。参考文献
1. David Dum, Noushin Taherpour, Anne Menz, Doris Höflmayer, Cosima Völkel, Andrea Hinsch, et al. Trophoblast Cell Surface Antigen 2 Expression in Human Tumors: A Tissue Microarray Study on 18,563 Tumors. Pathobiology. 2022;89(4):245-258. PMID: 35477165. doi: 10.1159/000522206.
2. Jixin Wang, Wen Yu, Rachel D'Anna, Anna Przybyla, Matt Wilson, Matthew Sung, et al. Pan-Cancer Proteomics Analysis to Identify Tumor-Enriched and Highly Expressed Cell Surface Antigens as Potential Targets for Cancer Therapeutics. Mol Cell Proteomics. 2023;22(9):100626. PMID: 37517589. doi: 10.1016/j.mcpro.2023.100626.
3. Genxiong Tang, Qi Tang, Lizhou Jia, Yuan Chen, Liangyuan Lin, Xingwang Kuai, et al. TROP2 increases growth and metastasis of human oral squamous cell carcinoma through activation of the PI3K/Akt signaling pathway. Int J Mol Med. 2019;44(6):2161-2170. PMID: 31638186. doi: 10.3892/ijmm.2019.4378.
4. Ying Xu, Wei Ren, Qingjian Li, Chaohui Duan, Xiaorong Lin, Zhuofei Bi, et al. LncRNA Uc003xsl.1-Mediated Activation of the NFκB/IL8 Axis Promotes Progression of Triple-Negative Breast Cancer. Cancer Res. 2022;82(4):556-570. PMID: 34965935. doi: 10.1158/0008-5472.CAN-21-1446.
5. Keiko Tanegashima, Yuka Tanaka, Takamichi Ito, Yoshinao Oda, Takeshi Nakahara TROP2 Expression and Therapeutic Implications in Cutaneous Squamous Cell Carcinoma: Insights From Immunohistochemical and Functional Analysis. Exp Dermatol. 2024;33(10):e15196. PMID: 39422290. doi: 10.1111/exd.15196.
6. Dan Morgenstern-Kaplan, Samuel A Kareff, Asaad Trabolsi, Estelamari Rodriguez, Harris Krause, Jennifer R Ribeiro, et al. Genomic, immunologic, and prognostic associations of TROP2 (TACSTD2) expression in solid tumors. Oncologist. 2024;29(11):e1480-e1491. PMID: 38986529. doi: 10.1093/oncolo/oyae168.
7. Yeonjin Jeon, Uiree Jo, Jongmoo Hong, Gyungyub Gong, Hee Jin Lee Trophoblast cell-surface antigen 2 (TROP2) expression in triple-negative breast cancer. BMC Cancer. 2022;22(1):1014. PMID: 36153494. doi: 10.1186/s12885-022-10076-7.
8. Hiromi Kobayashi, Yuko Minami, Yoichi Anami, Yuzuru Kondou, Tatsuo Iijima, Junko Kano, et al. Expression of the GA733 gene family and its relationship to prognosis in pulmonary adenocarcinoma. Virchows Arch. 2010;457(1):69-76. PMID: 20473768. doi: 10.1007/s00428-010-0930-8.
9. Dominic Fong, Gilbert Spizzo, Johanna M Gostner, Guenther Gastl, Patrizia Moser, Clemens Krammel, et al. TROP2: a novel prognostic marker in squamous cell carcinoma of the oral cavity. Mod Pathol. 2008;21(2):186-91. PMID: 18084248.
10. Michela Cortesi, Michele Zanoni, Roberta Maltoni, Sara Ravaioli, Maria Maddalena Tumedei, Francesca Pirini, et al. TROP2 (trophoblast cell-surface antigen 2): a drug target for breast cancer. Expert Opin Ther Targets. 2022;26(7):593-602. PMID: 35962580. doi: 10.1080/14728222.2022.2113513.
11. Annie R A McDougall, Stuart B Hooper, Valerie A Zahra, Foula Sozo, Camden Y Lo, Timothy J Cole, et al. The oncogene Trop2 regulates fetal lung cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2011;301(4):L478-89. PMID: 21743029. doi: 10.1152/ajplung.00063.2011.
12. Lizhou Jia, Tengqi Wang, Guipeng Ding, Xingwang Kuai, Xiaolei Wang, Bin Wang, et al. Trop2 inhibition of P16 expression and the cell cycle promotes intracellular calcium release in OSCC. Int J Biol Macromol. 2020;164:2409-2417. PMID: 32768481. doi: 10.1016/j.ijbiomac.2020.07.234.
13. Xia Sun, Lizhou Jia, Tengqi Wang, Yulian Zhang, Wei Zhao, Xiangcheng Wang, et al. Trop2 binding IGF2R induces gefitinib resistance in NSCLC by remodeling the tumor microenvironment. J Cancer. 2021;12(17):5310-5319. PMID: 34335947. doi: 10.7150/jca.57711.
14. Barbara A Frederick, Barbara A Helfrich, Christopher D Coldren, Di Zheng, Dan Chan, Paul A Bunn, et al. Epithelial to mesenchymal transition predicts gefitinib resistance in cell lines of head and neck squamous cell carcinoma and non-small cell lung carcinoma. Mol Cancer Ther. 2007;6(6):1683-91. PMID: 17541031.
15. Suzanne Chartier, Camille Brochard, Charlotte Martinat, Florence Coussy, Jean-Guillaume Feron, Youlia Kirova, et al. TROP2, androgen receptor, and PD-L1 status in histological subtypes of high-grade metaplastic breast carcinomas. Histopathology. 2023;82(5):664-671. PMID: 36527253. doi: 10.1111/his.14852.
16. Wenpeng Huang, Liming Li, Yuhan Zhou, Qi Yang, Jason C Mixdorf, Todd E Barnhart, et al. Preclinical evaluation of zirconium-89 labeled anti-Trop2 antibody-drug conjugate (Trodelvy) for imaging in gastric cancer and triple-negative breast cancer. Eur J Nucl Med Mol Imaging. 2025;52(7):2369-2383. PMID: 39878898. doi: 10.1007/s00259-025-07106-4.
17. Ryan K Rader, Carey K Anders, Nancy U Lin, Sarah L Sammons Available Systemic Treatments and Emerging Therapies for Breast Cancer Brain Metastases. Curr Treat Options Oncol. 2023;24(6):611-627. PMID: 37071254. doi: 10.1007/s11864-023-01086-z.
18. Ioannis P Trontzas, Mengni He, Anna Wurtz, Charles J Robbins, Nathaniel Robinson, Katherine Bates, et al. Quantitative Protein Expression of Antibody-Drug Conjugate Targets in EGFR Mutated and Wild-type Non-Small Cell Lung Cancer. Clin Cancer Res. 2025;31(13):2767-2776. PMID: 40047548. doi: 10.1158/1078-0432.CCR-24-3347.
19. Naohito Hashimoto, Shota Takei, Kyoichi Kaira, Hisao Imai, Kosuke Hashimoto, Yu Miura, et al. Prognostic relevance of TROP2 expression in patients with non-small cell lung cancer receiving immunotherapy. Sci Rep. 2025;15(1):35427. PMID: 41073519. doi: 10.1038/s41598-025-19362-3.
20. Chih-Jen Yang, Cheng-Hao Chuang, Yin-Wen Chen, Ching-Tang Huang, Pei-Hui Wang, Yen-Yi Zhen, et al. The complex interplay of TROP2 and PD-L1 in immune regulation and drug resistance in lung cancer. Am J Cancer Res. 2025;15(5):2413-2426. PMID: 40520853. doi: 10.62347/NHFJ1535.
21. Sára Lenárt, Peter Lenárt, Lucia Knopfová, Hana Kotasová, Vendula Pelková, Veronika Sedláková, et al. TACSTD2 upregulation is an early reaction to lung infection. Sci Rep. 2022;12(1):9583. PMID: 35688908. doi: 10.1038/s41598-022-13637-9.
22. Sebastian Foersch, Maxime Schmitt, Anne-Sophie Litmeyer, Markus Tschurtschenthaler, Thomas Gress, Detlef K Bartsch, et al. TROP2 in colorectal carcinoma: associations with histopathology, molecular phenotype, and patient prognosis. J Pathol Clin Res. 2024;10(5):e12394. PMID: 39177576. doi: 10.1002/2056-4538.12394.
23. Roberto Moretto, Marco Maria Germani, Mirella Giordano, Veronica Conca, Agnese Proietti, Cristina Niccoli, et al. Trop-2 and Nectin-4 immunohistochemical expression in metastatic colorectal cancer: searching for the right population for drugs' development. Br J Cancer. 2023;128(7):1391-1399. PMID: 36759721. doi: 10.1038/s41416-023-02180-7.
24. Lizhou Jia, Yuhao Fu, Ning Zhang, Yang Liu, Lin Su, Haisheng Wang, et al. Directional conjugation of Trop2 antibody to black phosphorus nanosheets for phototherapy in orthotopic gastric carcinoma. Nanomedicine. 2023;51:102687. PMID: 37121458. doi: 10.1016/j.nano.2023.102687.
25. Eleni Papacharisi, Alexandra C Braun, Marija Vranic, Andreas M Pahl, Torsten Hechler Novel Amanitin-Based Antibody-Drug Conjugates Targeting TROP2 for the Treatment of Pancreatic Cancer. Mol Cancer Ther. 2025;24(4):485-496. PMID: 39564769. doi: 10.1158/1535-7163.MCT-24-0266.
26. Caili Xu, Min Zhu, Qian Wang, Jiajun Cui, Yuping Huang, Xiting Huang, et al. TROP2-directed nanobody-drug conjugate elicited potent antitumor effect in pancreatic cancer. J Nanobiotechnology. 2023;21(1):410. PMID: 37932752. doi: 10.1186/s12951-023-02183-9.
27. Racha Hosni, Niklas Klümper, Christine Sanders, Sana Hosni, Vittorio Branchi, Alexander Semaan, et al. The Antibody-Drug Conjugate Sacituzumab Govitecan (IMMU-132) Represents a Potential Novel Therapeutic Strategy in Cholangiocarcinoma. Mol Cancer Ther. 2025. PMID: 40629695. doi: 10.1158/1535-7163.MCT-24-0972.
28. Racha Hosni, Niklas Klümper, Christine Sanders, Sana Hosni, Vittorio Branchi, Alexander Semaan, et al. The antibody-drug conjugate sacituzumab govitecan (IMMU-132) represents a potential novel therapeutic strategy in cholangiocarcinoma. Mol Cancer Ther. 2025. PMID: 40509933. doi: 10.1158/1535-7163.MCT-24-0972.
29. Jinru Yang, Fangyuan Zhang, Xing Cai, Zixuan Ding, Conghua Xie, Hong Qiu Trop2 Expression in Digestive Neoplasms: A Promising Target for Antibody-Drug Conjugates. Oncologist. 2025. PMID: 41100061. doi: 10.1093/oncolo/oyaf320.
30. Michael R Mallmann, Sina Tamir, Katharina Alfter, Dominik Ratiu, Alexander Quaas, Christian M Domroese Expression of Potential Antibody-Drug Conjugate Targets in Cervical Cancer. Cancers (Basel). 2024;16(9). PMID: 38730739. doi: 10.3390/cancers16091787.
31. Joanna M Porter, Cici Jin, Michael Churchman, Ian Croy, Catriona M Gourley, Oni Glyn-Wright, et al. Antibody drug conjugate targets are highly differentially expressed across the major types of ovarian cancer. Eur J Cancer. 2025;224:115522. PMID: 40446758. doi: 10.1016/j.ejca.2025.115522.
32. Victoria M Ettorre, Cem Demirkiran, Stefania Bellone, Na Niu, Natalia Buza, Namrata Sethi, et al. Preclinical activity of sacituzumab govitecan in TROP2-positive low-grade serous ovarian cancer patient-derived xenograft models. Int J Gynecol Cancer. 2025;35(9):101988. PMID: 40680453. doi: 10.1016/j.ijgc.2025.101988.
33. Blair McNamara, Michelle Greenman, Stefania Bellone, Luca A Santin, Cem Demirkiran, Levent Mutlu, et al. Preclinical activity of datopotamab deruxtecan, a novel TROP2 directed antibody-drug conjugate targeting trophoblast cell-surface antigen 2 (TROP2) in ovarian carcinoma. Gynecol Oncol. 2024;189:16-23. PMID: 38981151. doi: 10.1016/j.ygyno.2024.07.002.
34. Britt K Erickson, Brian Slomovitz, Matthew Powell, Ramez N Eskander Top advances of the year: Uterine cancer. Cancer. 2024;130(14):2409-2412. PMID: 38620054. doi: 10.1002/cncr.35321.
35. Giovanni Fucà, Ilaria Sabatucci, Mariachiara Paderno, Domenica Lorusso The clinical landscape of antibody-drug conjugates in endometrial cancer. Int J Gynecol Cancer. 2024;34(11):1795-1804. PMID: 39074933. doi: 10.1136/ijgc-2024-005607.
36. Giovanni Fucà, Ilaria Sabatucci, Mariachiara Paderno, Domenica Lorusso The clinical landscape of antibody-drug conjugates in endometrial cancer. Int J Gynecol Cancer. 2024;34(11):1795-1804. PMID: 40229038. doi: 10.1136/ijgc-2024-005607.
37. Chanhee Han, Emanuele Perrone, Burak Zeybek, Stefania Bellone, Joan Tymon-Rosario, Gary Altwerger, et al. In vitro and in vivo activity of sacituzumab govitecan, an antibody-drug conjugate targeting trophoblast cell-surface antigen 2 (Trop-2) in uterine serous carcinoma. Gynecol Oncol. 2020;156(2):430-438. PMID: 31839338. doi: 10.1016/j.ygyno.2019.11.018.
38. Frederik A Stuebs, Antje Knöll, Arndt Hartmann, Christian Matek, Nelson John, Lothar Häberle, et al. Trop2-expression in primary vulvar squamous cell carcinoma. Gynecol Oncol. 2025;196:78-84. PMID: 40184712. doi: 10.1016/j.ygyno.2025.03.049.
39. Anne Kathrin Höhn, Benjamin Wolf, Mirjam Forberger, Christine E Brambs, Blake Gilks, Lien Hoang, et al. Trop2 Expression in Correlation to the Molecular Subtype in Vulvar Squamous Cell Carcinomas. Pathobiology. 2025;92(3):150-156. PMID: 39837304. doi: 10.1159/000543554.
40. Veronika Bahlinger, Annalena Branz, Pamela L Strissel, Reiner Strick, Fabienne Lange, Carol I Geppert, et al. Associations of TACSTD2/TROP2 and NECTIN-4/NECTIN-4 with molecular subtypes, PD-L1 expression, and FGFR3 mutational status in two advanced urothelial bladder cancer cohorts. Histopathology. 2024;84(5):863-876. PMID: 38196202. doi: 10.1111/his.15130.
41. Jonathan Chou, Kai Trepka, Martin Sjöström, Emily A Egusa, Carissa E Chu, Jun Zhu, et al. TROP2 Expression Across Molecular Subtypes of Urothelial Carcinoma and Enfortumab Vedotin-resistant Cells. Eur Urol Oncol. 2022;5(6):714-718. PMID: 35216942. doi: 10.1016/j.euo.2021.11.005.
42. Jan H Müller, Henning Plage, Sefer Elezkurtaj, Tim Mandelkow, Zhihao Huang, Magalie C J Lurati, et al. Loss of TROP2 and epithelial cell adhesion molecule expression is linked to grade progression in pTa but unrelated to disease outcome in pT2-4 urothelial bladder carcinomas. Front Oncol. 2023;13:1342367. PMID: 38282671. doi: 10.3389/fonc.2023.1342367.
43. Takamichi Ito, Yuka Tanaka, Dai Ogata, Haruto Nishida, Tatsushi Shiomi, Ryo Tanaka, et al. A multicenter study on TROP2 as a potential targeted therapy for extramammary Paget disease in Japan. Sci Rep. 2025;15(1):409. PMID: 39747638. doi: 10.1038/s41598-024-84566-y.
44. Takamichi Ito, Keiko Tanegashima, Yuka Tanaka, Hiroki Hashimoto, Maho Murata, Yoshinao Oda, et al. Trop2 Expression in Extramammary Paget's Disease and Normal Skin. Int J Mol Sci. 2021;22(14). PMID: 34299325. doi: 10.3390/ijms22147706.
45. Dan-Dan Zhou, Li-Ping Sun, Qun Yu, Xiao-Tian Zhai, Lan-Wen Zhang, Rui-Juan Gao, et al. Elucidating the development, characterization, and antitumor potential of a novel humanized antibody against Trop2. Int J Biol Macromol. 2023;253(Pt 6):127105. PMID: 37769779. doi: 10.1016/j.ijbiomac.2023.127105.
46. Li-Ping Sun, Wei-Qi Bai, Dan-Dan Zhou, Xiao-Fan Wu, Lan-Wen Zhang, A-Long Cui, et al. hIMB1636-MMAE, a Novel TROP2-Targeting Antibody-Drug Conjugate Exerting Potent Antitumor Efficacy in Pancreatic Cancer. J Med Chem. 2023;66(21):14700-14715. PMID: 37883180. doi: 10.1021/acs.jmedchem.3c01210.
47. Huicheng Liu, Lili Bai, Liu Huang, Na Ning, Lin Li, Yijia Li, et al. Bispecific antibody targeting TROP2xCD3 suppresses tumor growth of triple negative breast cancer. J Immunother Cancer. 2021;9(10). PMID: 34599021. doi: 10.1136/jitc-2021-003468.
48. Caili Luo, Anni Ren, Zixuan Jin, Jianxin Zhang, Wei Shi, Yue Zeng, et al. Design and synthesis of novel site-specific antibody-drug conjugates that target TROP2. Bioorg Med Chem. 2024;110:117828. PMID: 38981219. doi: 10.1016/j.bmc.2024.117828.
49. Jayakumar R Nair, Tzu-Ting Huang, Anu Sunkara, Margaret R Pruitt, Kristen R Ibanez, Chih-Yuan Chiang, et al. Distinct effects of sacituzumab govitecan and berzosertib on DNA damage response in ovarian cancer. iScience. 2024;27(12):111283. PMID: 39628575. doi: 10.1016/j.isci.2024.111283.
50. Daisuke Okajima, Satoru Yasuda, Takanori Maejima, Tsuyoshi Karibe, Ken Sakurai, Tetsuo Aida, et al. Datopotamab Deruxtecan, a Novel TROP2-directed Antibody-drug Conjugate, Demonstrates Potent Antitumor Activity by Efficient Drug Delivery to Tumor Cells. Mol Cancer Ther. 2021;20(12):2329-2340. PMID: 34413126. doi: 10.1158/1535-7163.MCT-21-0206.
51. Rachel M DeVay, Kathy Delaria, Guoyun Zhu, Charles Holz, Davide Foletti, Janette Sutton, et al. Improved Lysosomal Trafficking Can Modulate the Potency of Antibody Drug Conjugates. Bioconjug Chem. 2017;28(4):1102-1114. PMID: 28151644. doi: 10.1021/acs.bioconjchem.7b00013.
52. Michelle Greenman, Stefania Bellone, Cem Demirkiran, Tobias Max Philipp Hartwich, Victoria M Ettorre, Blair McNamara, et al. Datopotamab deruxtecan, a novel TROP2-tareting antibody-drug conjugate with a topoisomerase I inhibitor payload, shows preclinical activity against primary and metastatic uterine and ovarian TROP2 over-expressing carcinosarcoma. Gynecol Oncol. 2025;199:64-71. PMID: 40582040. doi: 10.1016/j.ygyno.2025.06.017.
53. Sabrina Forveille, Marion Leduc, Allan Sauvat, Guido Kroemer, Oliver Kepp Datopotamab deruxtecan induces hallmarks of immunogenic cell death. Cell Stress. 2025;9:194-200. PMID: 40800161. doi: 10.15698/cst2025.08.311.
54. Aiko Yamaguchi, Chisato M Yamazaki, Yasuaki Anami, Summer Y Y Ha, Wei Xiong, Robert T Ta, et al. 177Lu-Labeled Antibody-Drug Conjugate: A Dual-Mechanistic Treatment Modality in Solid Tumors. Mol Cancer Ther. 2025;24(6):907-919. PMID: 40051166. doi: 10.1158/1535-7163.MCT-24-0254.
55. L M Spring, S M Tolaney, G Fell, V Bossuyt, R O Abelman, B Wu, et al. Response-guided neoadjuvant sacituzumab govitecan for localized triple-negative breast cancer: results from the NeoSTAR trial. Ann Oncol. 2024;35(3):293-301. PMID: 38092228. doi: 10.1016/j.annonc.2023.11.018.
56. Laila C Roisman, Shir Mann, Afifi Basel, Ranin Marei, Belal Krayim, Gleb Kornev, et al. The PESGA Trial: A Prospective, Open-Label, Single-Arm, Phase II Study to Evaluate First Line Therapy for Extensive-Stage Small Cell Lung Cancer (ES-SCLC) Patients, Treated by Induction Carboplatin/Etoposide/Pembrolizumab Followed by Maintenance of Pembrolizumab/ Sacituzumab Govitecan. Clin Lung Cancer. 2025;26(4):267-270. PMID: 40064573. doi: 10.1016/j.cllc.2025.02.002.
57. Sascha Hoppe, Lydia Meder, Florian Gebauer, Roland T Ullrich, Thomas Zander, Axel M Hillmer, et al. Trophoblast Cell Surface Antigen 2 (TROP2) as a Predictive Bio-Marker for the Therapeutic Efficacy of Sacituzumab Govitecan in Adenocarcinoma of the Esophagus. Cancers (Basel). 2022;14(19). PMID: 36230712. doi: 10.3390/cancers14194789.
58. Cristina Nieto-Jiménez, Adrián Sanvicente, Cristina Díaz-Tejeiro, Víctor Moreno, Alfonso Lopez de Sá, Emiliano Calvo, et al. Uncovering therapeutic opportunities in the clinical development of antibody-drug conjugates. Clin Transl Med. 2023;13(9):e1329. PMID: 37740463. doi: 10.1002/ctm2.1329.
59. Toshio Shimizu, Jacob Sands, Kiyotaka Yoh, Alexander Spira, Edward B Garon, Satoru Kitazono, et al. First-in-Human, Phase I Dose-Escalation and Dose-Expansion Study of Trophoblast Cell-Surface Antigen 2-Directed Antibody-Drug Conjugate Datopotamab Deruxtecan in Non-Small-Cell Lung Cancer: TROPION-PanTumor01. J Clin Oncol. 2023;41(29):4678-4687. PMID: 37327461. doi: 10.1200/JCO.23.00059.
60. Neel Belani, Anjali Donnelly, Alexander Spira An evaluation of datopotamab deruxtecan for the treatment of non-small cell lung cancer. Expert Opin Biol Ther. 2025;25(7):695-701. PMID: 40515573. doi: 10.1080/14712598.2025.2519532.
61. Yasong Lu, Shuang Liang, Ying Hong, Naoyuki Tajima, Kashyap Patel, Hanbin Li, et al. Application of the model-informed drug development paradigm to datopotamab deruxtecan dose selection for late-stage development. CPT Pharmacometrics Syst Pharmacol. 2024;13(1):23-28. PMID: 37915242. doi: 10.1002/psp4.13058.
62. J Wang, Y Zhang, R Bai, Y Wu, Z Tong, A Liu, et al. Novel TROP2 antibody-drug conjugates for treatment of HER2-negative metastatic breast cancer patients with brain metastases: a promising option. ESMO Open. 2025;10(5):105059. PMID: 40359710. doi: 10.1016/j.esmoop.2025.105059.
63. Funda Meric-Bernstam, Erkan Yuca, Kurt W Evans, Ming Zhao, Takanori Maejima, Tsuyoshi Karibe, et al. Antitumor Activity and Biomarker Analysis for TROP2 Antibody-Drug Conjugate Datopotamab Deruxtecan in Patient-Derived Breast Cancer Xenograft Models. Clin Cancer Res. 2025;31(3):573-587. PMID: 39585341. doi: 10.1158/1078-0432.CCR-24-1948.
64. Victoria M Ettorre, Cem Demirkiran, Stefania Bellone, Tobias Max Philipp Hartwich, Michelle Greenman, Blair McNamara, et al. Effective preclinical activity of datopotamab deruxtecan (Dato-DXd), an ADC targeting trophoblast cell-surface antigen 2 (TROP2), against primary cervical carcinoma cell lines and xenografts. Gynecol Oncol. 2025;201:195-202. PMID: 40907200. doi: 10.1016/j.ygyno.2025.08.027.
65. Jiani Wang, Zhongsheng Tong, Yinuo Tan, Yehui Shi, Yun Wu, Qing Zhou, et al. Phase 1a study of ESG401, a Trop2 antibody-drug conjugate, in patients with locally advanced/metastatic solid tumors. Cell Rep Med. 2024;5(9):101707. PMID: 39216478. doi: 10.1016/j.xcrm.2024.101707.
66. Ting-Yu Chang, Chun-Jung Lin, Shih-Ni Wen, Yi-Chen Wu, Cheng-Yen Wei, Jye-Yu Huang, et al. Preclinical evaluation of a novel antibody-drug conjugate OBI-992 for Cancer therapy. Sci Rep. 2025;15(1):8735. PMID: 40082588. doi: 10.1038/s41598-025-92697-z.
67. Wan-Fen Li, Ming-Feng Chiang, Hao-Cheng Weng, Jhih-Jie Yang, Hsin-Shan Wu, Szu-Yu Wu, et al. OBI-992, a Novel TROP2-Targeted Antibody-Drug Conjugate, Demonstrates Antitumor Activity in Multiple Cancer Models. Mol Cancer Ther. 2025;24(2):163-175. PMID: 39786401. doi: 10.1158/1535-7163.MCT-24-0588.
68. Chi-Sheng Shia, Shih-Ni Wen, Ren-Yu Hsu, Jyy-Shiuan Tu, Hui-Wen Chang, Hao-Cheng Weng, et al. Preclinical pharmacokinetic, pharmacodynamic, and safety profile of OBI-992: a novel TROP2-targeted antibody-drug conjugate. Mol Cancer Ther. 2025. PMID: 40762235. doi: 10.1158/1535-7163.MCT-24-1176.
69. Yezhe Cheng, Xiaoxi Yuan, Qiang Tian, Xiuying Huang, Yang Chen, Yuzhi Pu, et al. Preclinical profiles of SKB264, a novel anti-TROP2 antibody conjugated to topoisomerase inhibitor, demonstrated promising antitumor efficacy compared to IMMU-132. Front Oncol. 2022;12:951589. PMID: 36620535. doi: 10.3389/fonc.2022.951589.
70. Elliott J Brea, Simon Baldacci, Neil Savage, Francesco Facchinetti, Conor Hinchey, Sachiv Chakravarti, et al. Systematic engineering of TROP2-targeted CAR T-cell therapy overcomes resistance pathways in solid tumors. Cancer Immunol Res. 2025. PMID: 40920095. doi: 10.1158/2326-6066.CIR-25-0527.
71. Chalermchai Somboonpatarakun, Nattaporn Phanthaphol, Kwanpirom Suwanchiwasiri, Boonyanuch Ramwarungkura, Pornpimon Yuti, Naravat Poungvarin, et al. Cytotoxicity of fourth-generation anti-Trop2 CAR-T cells against breast cancer. Int Immunopharmacol. 2024;129:111631. PMID: 38359664. doi: 10.1016/j.intimp.2024.111631.
72. Shiyu Sun, Xiaojia Wang, Yongchen Chen, Ziwei Liang, Zuomin Nian, Wanni Xu, et al. Preclinical evaluation of antitumor activity and toxicity of TROP2-specific CAR-T cells for treatment of triple-negative breast cancer. J Immunother Cancer. 2025;13(9). PMID: 40903191. doi: 10.1136/jitc-2025-012442.
73. Wenjing Li, Tian Gao, Renjun Pei Selection of trophoblast cell surface antigen 2-targeted aptamer for the development of cytotoxic aptamer-drug conjugate. Int J Biol Macromol. 2024;279(Pt 4):135456. PMID: 39250993. doi: 10.1016/j.ijbiomac.2024.135456.
74. Yajie Deng, Zi Hui, Lu Liu, Fei Gao, Xuan Chen, Lulu Huang, et al. Modular assembly and evaluation of a TROP2-targeting immunotoxin for cancer therapy. Int J Biol Macromol. 2025;310(Pt 2):143311. PMID: 40253026. doi: 10.1016/j.ijbiomac.2025.143311.
75. Yining Sun, Zhixin Hao, Hannan Gao, Guangjie Yang, Bo Pan, Min Zhu, et al. [. J Nucl Med. 2025;66(4):543-551. PMID: 39947911. doi: 10.2967/jnumed.124.268564.
76. Wei Huang, Min Cao, Yanfei Wu, You Zhang, Shuxian An, Xinbing Pan, et al. Immuno-PET/CT Imaging of Trop2 with [. J Nucl Med. 2024;65(12):1904-1910. PMID: 39542697. doi: 10.2967/jnumed.124.268751.
77. Shigehiro Koganemaru, Hirobumi Fuchigami, Chihiro Morizono, Hiroko Shinohara, Yasutoshi Kuboki, Keiji Furuuchi, et al. Potential Mechanisms of Interstitial Lung Disease Induced by Antibody-Drug Conjugates Based on Quantitative Analysis of Drug Distribution. Mol Cancer Ther. 2025;24(2):242-250. PMID: 39450538. doi: 10.1158/1535-7163.MCT-24-0267.
78. Rebecca S Heist, Jacob Sands, Aditya Bardia, Toshio Shimizu, Aaron Lisberg, Ian Krop, et al. Clinical management, monitoring, and prophylaxis of adverse events of special interest associated with datopotamab deruxtecan. Cancer Treat Rev. 2024;125:102720. PMID: 38502995. doi: 10.1016/j.ctrv.2024.102720.
79. Ilana Schlam, Paolo Tarantino, Sara M Tolaney Managing adverse events of sacituzumab govitecan. Expert Opin Biol Ther. 2023;23(11):1103-1111. PMID: 37800595. doi: 10.1080/14712598.2023.2267975.
80. James T Coates, Sheng Sun, Ignaty Leshchiner, Nayana Thimmiah, Elizabeth E Martin, Daniel McLoughlin, et al. Parallel Genomic Alterations of Antigen and Payload Targets Mediate Polyclonal Acquired Clinical Resistance to Sacituzumab Govitecan in Triple-Negative Breast Cancer. Cancer Discov. 2021;11(10):2436-2445. PMID: 34404686. doi: 10.1158/2159-8290.CD-21-0702.
81. Sally M Shalaby, Salma A Shawky, Hassan Ashour, Walaa Sarhan The interplay between COX-2, chemotherapeutic drugs, and chemoresistance in colon cancer. Sci Rep. 2025;15(1):15837. PMID: 40328989. doi: 10.1038/s41598-025-98451-9.
82. Yves Pommier, Anish Thomas New Life of Topoisomerase I Inhibitors as Antibody-Drug Conjugate Warheads. Clin Cancer Res. 2023;29(6):991-993. PMID: 36637483. doi: 10.1158/1078-0432.CCR-22-3640.
83. Tao Li, Xianqiang Yu, Xinyao Wan, Jing Liu, Jie Zheng, Ziyu Sun, et al. Exploiting synthetic lethality in PDAC with antibody drug conjugates and ATR inhibition. Eur J Med Chem. 2025;286:117305. PMID: 39874630. doi: 10.1016/j.ejmech.2025.117305.
84. Ming Zhao, Timothy P DiPeri, Maria Gabriela Raso, Xiaofeng Zheng, Yasmeen Qamar Rizvi, Kurt W Evans, et al. Epigenetically upregulating TROP2 and SLFN11 enhances therapeutic efficacy of TROP2 antibody drug conjugate sacitizumab govitecan. NPJ Breast Cancer. 2023;9(1):66. PMID: 37567892. doi: 10.1038/s41523-023-00573-8.
85. Daniel V Santi, Gary W Ashley, Luc Cabel, Francois-Clement Bidard Could a Long-Acting Prodrug of SN-38 be Efficacious in Sacituzumab Govitecan-Resistant Tumors?. BioDrugs. 2024;38(2):171-176. PMID: 38236523. doi: 10.1007/s40259-024-00643-8.
86. Caili Xu, Xiting Huang, Qinchao Hu, Wenjing Xue, Kaicheng Zhou, Xingxiu Li, et al. Modulating autophagy to boost the antitumor efficacy of TROP2-directed antibody-drug conjugate in pancreatic cancer. Biomed Pharmacother. 2024;180:117550. PMID: 39418963. doi: 10.1016/j.biopha.2024.117550.
87. Giulia Cursano, Alberto Concardi, Mariia Ivanova, Chiara Frascarelli, Eltjona Mane, Elisa Mangione, et al. Inter-Assay Variability of TROP2 Immunohistochemistry in Triple-Negative Breast Cancer. Mol Diagn Ther. 2025. PMID: 40914741. doi: 10.1007/s40291-025-00814-5.
88. Yusuke Sayama, Mika K Kaneko, Yukinari Kato Development and characterization of TrMab‑6, a novel anti‑TROP2 monoclonal antibody for antigen detection in breast cancer. Mol Med Rep. 2021;23(2). PMID: 33300065. doi: 10.3892/mmr.2020.11731.
89. Tomohiro Tanaka, Tomokazu Ohishi, Teizo Asano, Junko Takei, Ren Nanamiya, Hideki Hosono, et al. An anti‑TROP2 monoclonal antibody TrMab‑6 exerts antitumor activity in breast cancer mouse xenograft models. Oncol Rep. 2021;46(1). PMID: 34013368. doi: 10.3892/or.2021.8083.
90. Charles J Robbins, Mengni He, Nay Chan, Revekka Khaimova, Katherine Bates, Ioannis P Trontzas, et al. Quantitative Multiplex Immunofluorescence Assay for Trophoblast Cell-Surface Antigen 2 and Human Epidermal Growth Factor Receptor 2 Expression in Breast Cancer: Toward Guiding Patient Selection for Antibody-Drug Conjugate Therapies. JCO Precis Oncol. 2025;9:e2500128. PMID: 40669019. doi: 10.1200/PO-25-00128.
91. S Morganti, R J Kusmick, M E Hughes, F Brasó-Maristany, K Smith, P Tarantino, et al. Survival outcomes and the role of DNADX ctDNA testing in patients treated with sacituzumab govitecan for metastatic breast cancer. ESMO Open. 2025;10(10):105828. PMID: 41033284. doi: 10.1016/j.esmoop.2025.105828.
抗体药物偶联物
2025-10-23
根据美国Clinicaltrial数据库数据,今年9月份,全球新开由企业资本主导的临床试验总数为826项,数量较8月份上升18.51%;单月新开临床试验数量高于去年同期水平,同比上升28.46%。
热门领域分布
从9月份新开临床试验热门适应证来看,乳腺癌为最主要的热门研发领域,新开临床试验31项,环比上涨72.22%,同比上涨93.75%。其次为非小细胞肺癌,新开临床试验22项,与去年同期相比上涨10.00%。值得注意的是,9月份新开临床试验数量上升幅度最大的热门适应证为结肠炎,由8月份的3项上升至9项,上升幅度为200.00%;数量下降幅度最大的适应证为卵巢癌,由8月份的13项下降到9项,下降幅度为30.77%。
对新开临床试验的发起企业进行统计后发现,9月份发起临床试验最多的企业为阿斯利康,新开临床试验16项,环比上升6.67%,同比上升45.45%。其次为辉瑞,新开临床试验11项,环比下降21.42%,同比增长83.33%。值得注意的是,9月份我国药企齐鲁制药和康诺亚生物进入新开临床试验数量排名前十榜单,分别新开8项和5项临床试验。其中,齐鲁制药为新开临床试验数量上升幅度最大的企业,与8月份相比上升了7倍。
对临床试验的申请国家和地区进行统计后发现,9月份美国仍为临床试验开展最为主要的国家,新开临床试验227项,相较8月份上涨了1.79%。其次是中国,新开临床试验数量为113项,与8月份相比下降了21.53%。
头部企业表现
9月份新开临床试验数量排名前三的企业分别为阿斯利康、辉瑞和百时美施贵宝,数量分别为16项、11项、10项。
阿斯利康9月份新开的16项临床试验中,有两项为Ⅲ期临床试验,适应证均为肿瘤。其中一项的适应证为尿路上皮癌(NCT07205822),试验药物为Dato-DXd(德达博妥单抗)。该药是一种靶向TROP2的抗体偶联药物(ADC),主要用于治疗乳腺癌和非小细胞肺癌。另一款进入Ⅲ期临床试验的药物为Durvalumab,适应证为硬纤维瘤(NCT06992609)。Durvalumab是一种人源化的抗PD-L1蛋白单克隆抗体,可阻断PD-L1与PD-1和CD80结合的能力。
辉瑞9月份新开的11项临床试验中,有3项为Ⅲ期临床试验,其中两项的适应证均为肿瘤,分别为泛晚期肿瘤(NCT06103734)和广泛性小细胞肺癌(NCT07028853)。其中,泛晚期肿瘤的试验药物为Zavegepant,该药属于第三代高选择性降钙素基因相关肽(CGRP)受体拮抗剂,通过阻断CGRP信号通路发挥治疗作用;广泛性小细胞肺癌的试验药物为Mevrometostat,是一款选择性EZH2抑制剂。另一项Ⅲ期临床试验的适应证为体外膜氧合并发症(NCT07029828),试验药物为Ritlecitinib,该药是一种口服选择性JAK3抑制剂。
百时美施贵宝9月份新开的10项临床试验中,有3项为Ⅲ期临床试验,适应证分别为多发性硬化症(NCT07140913)、肾结石(NCT07116967)和克罗恩病(NCT06979453)。以多发性硬化症为适应证的试验药物为Xanomeline/Trospium Chloride,这是一款复方药物。其中,Xanomeline通过激活中枢神经系统的M1/M4受体,改善精神分裂症的阳性(如幻觉)、阴性(如情感淡漠)及认知症状;Trospium Chloride作为外周拮抗剂,抑制Xanomeline对胃肠道的副作用(如恶心、呕吐),提高机体耐受性。以肾结石和克罗恩病为适应证的试验药物均为Deucravacitinib,该药是一种选择性酪氨酸激酶2(TYK2)变构抑制剂,通过选择性结合TYK2假激酶结构域,抑制IL-12/23和Ⅰ型干扰素信号通路,从而调节免疫反应。
齐鲁制药9月份新开8项临床试验,其中3项为Ⅲ期临床试验,适应证分别为周围神经病变(NCT07162883)、胰腺癌(NCT07079228)和特应性皮炎(NCT06732323)。以周围神经病变为适应证的试验药物为QL2107,这是一款PD-1抑制剂生物类似药。以胰腺癌为适应证的试验药物为QLS31905,该药是齐鲁制药自主研发的靶向Claudin18.2(CLDN18.2)和CD3的双特异性T细胞衔接器(BiTE),通过结合肿瘤细胞表面的CLDN18.2和T细胞表面的CD3,激活T细胞,从而杀伤肿瘤细胞。以特应性皮炎为适应证的试验药物为ESG401,该药是一款靶向 CLDN18.2的单克隆抗体,主要用于治疗CLDN18.2阳性实体瘤。
康诺亚生物9月份新开的5项临床试验中,包括两项Ⅲ期临床试验,适应证分别为多发性硬化症(NCT07181239)和特应性皮炎(NCT07106372)。多发性硬化症的试验药物为CM336,该药是康诺亚生物自主研发的一款靶向BCMA和CD3的双特异性抗体,通过同时结合靶细胞上的BCMA和T细胞上的CD3,激活T细胞介导的细胞毒性(TDCC)作用,从而清除靶细胞。以特应性皮炎为适应证的试验药物为Stapokibatrt,该药是一款靶向I L-4Rα的单克隆抗体,主要用于治疗2型炎症性疾病。
(数据来源于美国Clinicaltrial数据库,统计时间为10月9日)
来源/ 中国医药报
文/ 陈宇哲
新媒体编辑:李佳欢
统筹策划:何红梅
中国医药报社版权所有,未经许可不得转载使用。
临床结果临床3期抗体药物偶联物临床失败
2025-10-22
·新浪看点
本文转自:中国医药报9月份美国Clinicaltrial数据库临床试验数据显示——乳腺癌药物研发最为活跃□ 陈宇哲根据美国Clinicaltrial数据库数据,今年9月份,全球新开由企业资本主导的临床试验总数为826项,数量较8月份上升18.51%;单月新开临床试验数量高于去年同期水平,同比上升28.46%。热门领域分布从9月份新开临床试验热门适应证来看,乳腺癌为最主要的热门研发领域,新开临床试验31项,环比上涨72.22%,同比上涨93.75%。其次为非小细胞肺癌,新开临床试验22项,与去年同期相比上涨10.00%。值得注意的是,9月份新开临床试验数量上升幅度最大的热门适应证为结肠炎,由8月份的3项上升至9项,上升幅度为200.00%;数量下降幅度最大的适应证为卵巢癌,由8月份的13项下降到9项,下降幅度为30.77%。(详见表1)对新开临床试验的发起企业进行统计后发现,9月份发起临床试验最多的企业为阿斯利康,新开临床试验16项,环比上升6.67%,同比上升45.45%。其次为辉瑞,新开临床试验11项,环比下降21.42%,同比增长83.33%。值得注意的是,9月份我国药企齐鲁制药和康诺亚生物进入新开临床试验数量排名前十榜单,分别新开8项和5项临床试验。其中,齐鲁制药为新开临床试验数量上升幅度最大的企业,与8月份相比上升了7倍。(详见表2)对临床试验的申请国家和地区进行统计后发现,9月份美国仍为临床试验开展最为主要的国家,新开临床试验227项,相较8月份上涨了1.79%。其次是中国,新开临床试验数量为113项,与8月份相比下降了21.53%。头部企业表现9月份新开临床试验数量排名前三的企业分别为阿斯利康、辉瑞和百时美施贵宝,数量分别为16项、11项、10项。阿斯利康9月份新开的16项临床试验中,有两项为Ⅲ期临床试验,适应证均为肿瘤。其中一项的适应证为尿路上皮癌(NCT07205822),试验药物为Dato-DXd(德达博妥单抗)。该药是一种靶向TROP2的抗体偶联药物(ADC),主要用于治疗乳腺癌和非小细胞肺癌。另一款进入Ⅲ期临床试验的药物为Durvalumab,适应证为硬纤维瘤(NCT06992609)。Durvalumab是一种人源化的抗PD-L1蛋白单克隆抗体,可阻断PD-L1与PD-1和CD80结合的能力。辉瑞9月份新开的11项临床试验中,有3项为Ⅲ期临床试验,其中两项的适应证均为肿瘤,分别为泛晚期肿瘤(NCT06103734)和广泛性小细胞肺癌(NCT07028853)。其中,泛晚期肿瘤的试验药物为Zavegepant,该药属于第三代高选择性降钙素基因相关肽(CGRP)受体拮抗剂,通过阻断CGRP信号通路发挥治疗作用;广泛性小细胞肺癌的试验药物为Mevrometostat,是一款选择性EZH2抑制剂。另一项Ⅲ期临床试验的适应证为体外膜氧合并发症(NCT07029828),试验药物为Ritlecitinib,该药是一种口服选择性JAK3抑制剂。百时美施贵宝9月份新开的10项临床试验中,有3项为Ⅲ期临床试验,适应证分别为多发性硬化症(NCT07140913)、肾结石(NCT07116967)和克罗恩病(NCT06979453)。以多发性硬化症为适应证的试验药物为Xanomeline/Trospium Chloride,这是一款复方药物。其中,Xanomeline通过激活中枢神经系统的M1/M4受体,改善精神分裂症的阳性(如幻觉)、阴性(如情感淡漠)及认知症状;Trospium Chloride作为外周拮抗剂,抑制Xanomeline对胃肠道的副作用(如恶心、呕吐),提高机体耐受性。以肾结石和克罗恩病为适应证的试验药物均为Deucravacitinib,该药是一种选择性酪氨酸激酶2(TYK2)变构抑制剂,通过选择性结合TYK2假激酶结构域,抑制IL-12/23和Ⅰ型干扰素信号通路,从而调节免疫反应。齐鲁制药9月份新开8项临床试验,其中3项为Ⅲ期临床试验,适应证分别为周围神经病变(NCT07162883)、胰腺癌(NCT07079228)和特应性皮炎(NCT06732323)。以周围神经病变为适应证的试验药物为QL2107,这是一款PD-1抑制剂生物类似药。以胰腺癌为适应证的试验药物为QLS31905,该药是齐鲁制药自主研发的靶向Claudin18.2(CLDN18.2)和CD3的双特异性T细胞衔接器(BiTE),通过结合肿瘤细胞表面的CLDN18.2和T细胞表面的CD3,激活T细胞,从而杀伤肿瘤细胞。以特应性皮炎为适应证的试验药物为ESG401,该药是一款靶向 CLDN18.2的单克隆抗体,主要用于治疗CLDN18.2阳性实体瘤。康诺亚生物9月份新开的5项临床试验中,包括两项Ⅲ期临床试验,适应证分别为多发性硬化症(NCT07181239)和特应性皮炎(NCT07106372)。多发性硬化症的试验药物为CM336,该药是康诺亚生物自主研发的一款靶向BCMA和CD3的双特异性抗体,通过同时结合靶细胞上的BCMA和T细胞上的CD3,激活T细胞介导的细胞毒性(TDCC)作用,从而清除靶细胞。以特应性皮炎为适应证的试验药物为Stapokibatrt,该药是一款靶向I L-4Rα的单克隆抗体,主要用于治疗2型炎症性疾病。(数据来源于美国Clinicaltrial数据库,统计时间为10月9日)
发布于:北京
临床结果临床3期抗体药物偶联物
100 项与 ESG-401 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 晚期三阴性乳腺癌 | 临床3期 | 中国 | 2025-08-27 | |
| HR阳性/HER2阴性乳腺癌 | 临床3期 | 中国 | 2024-06-25 | |
| HR阳性/HER2阴性乳腺癌 | 临床3期 | 中国 | 2024-06-25 | |
| 膀胱癌 | 临床2期 | 中国 | 2021-09-14 | |
| 结直肠癌 | 临床2期 | 中国 | 2021-09-14 | |
| 非小细胞肺癌 | 临床2期 | 中国 | 2021-09-14 | |
| 卵巢癌 | 临床2期 | 中国 | 2021-09-14 | |
| 胃癌 | 临床2期 | 中国 | 2021-09-14 | |
| 三阴性乳腺癌 | 临床2期 | 中国 | 2021-09-14 | |
| 实体瘤 | 临床2期 | 中国 | 2021-09-09 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
临床1期 | 转移性HER2阴性乳腺癌 HER2 Negative | 17 | 夢淵衊艱衊艱襯餘膚壓(獵範醖網獵鬱夢選鑰膚) = 膚壓醖鏇憲膚鏇遞觸積 積襯遞獵製遞夢網醖蓋 (廠鹽艱遞窪鏇繭淵網築, 27.8 ~ 77.0) 更多 | 积极 | 2025-05-13 | ||
临床1期 | 150 | (previously untreated TNBC) | 醖製鹽繭遞鏇鬱窪鑰艱(夢鹹願艱網憲獵繭襯範) = 廠鏇選鹽衊窪願簾製廠 壓簾餘願網築鬱願餘築 (齋淵膚艱鬱築構獵襯餘 ) 更多 | 积极 | 2025-01-29 | ||
(late-stage TNBC) | 醖製鹽繭遞鏇鬱窪鑰艱(夢鹹願艱網憲獵繭襯範) = 顧醖遞選繭遞簾醖艱窪 壓簾餘願網築鬱願餘築 (齋淵膚艱鬱築構獵襯餘 ) 更多 | ||||||
临床1期 | 117 | ESG401tage HR+/HER2-BC 12 mg/kg | 糧簾窪鏇範糧範願獵鑰(觸鏇襯糧淵構齋艱鹹艱) = 積衊鑰遞鹹觸繭築餘蓋 糧壓淵餘獵選網餘夢餘 (積積夢鏇蓋鹽壓觸獵憲, 64.7 ~ 87.5) 更多 | 积极 | 2024-09-16 | ||
ESG401tage TNBC 12 mg/kg | 糧簾窪鏇範糧範願獵鑰(觸鏇襯糧淵構齋艱鹹艱) = 廠衊壓壓壓範憲衊齋艱 糧壓淵餘獵選網餘夢餘 (積積夢鏇蓋鹽壓觸獵憲, 44.8 ~ 77.5) 更多 | ||||||
临床1期 | 16 | ESG401 12 mg/kg | 襯築膚鏇醖淵獵齋顧範(糧膚艱構範醖衊餘遞範) = 憲夢觸餘淵觸憲膚構築 齋窪鏇積糧製憲窪製膚 (積憲夢鏇醖繭窪築範製, 15.2 ~ 64.6) 更多 | 积极 | 2024-09-15 | ||
临床1期 | 40 | 夢廠顧網製鹹觸遞蓋積(淵醖製襯積鑰壓積窪廠) = 襯糧製積壓壓壓願餘壓 獵壓憲齋構願齋網襯餘 (壓獵鑰憲壓鹽襯遞蓋襯 ) 更多 | 积极 | 2024-08-30 | |||
(TRD) | 夢廠顧網製鹹觸遞蓋積(淵醖製襯積鑰壓積窪廠) = 簾蓋遞壓衊餘鹹醖鏇鑰 獵壓憲齋構願齋網襯餘 (壓獵鑰憲壓鹽襯遞蓋襯 ) 更多 | ||||||
临床1期 | 三阴性乳腺癌 一线 | 19 | 範夢鬱襯醖糧齋範壓鬱(積醖簾衊齋鬱鏇夢鹽築) = 簾衊範襯願鹽壓選觸夢 齋糧夢夢觸蓋壓鏇獵願 (網鏇簾簾艱淵蓋夢醖餘 ) 更多 | 积极 | 2024-08-09 | ||
临床1期 | 23 | ESG401 (16 mg/kg IV) | 範製簾夢鑰壓鹹齋願獵(鏇壓積醖糧淵網艱範齋) = 蓋願製顧憲鑰構鑰築襯 齋蓋鏇窪夢窪顧憲願遞 (築繭鬱簾蓋餘糧顧鏇獵 ) 更多 | 积极 | 2024-05-24 | ||
临床1/2期 | 35 | (triple negative breast cancer) | 範淵鏇構鑰遞製鏇構夢(顧廠鏇觸鑰獵鹹築廠築) = 襯選製構觸襯衊醖糧淵 餘簾壓鏇醖鏇壓簾蓋構 (鹽憲鑰積網衊範製顧夢 ) 更多 | 积极 | 2023-05-26 | ||
(HR+/HER2- breast cancer) | 範淵鏇構鑰遞製鏇構夢(顧廠鏇觸鑰獵鹹築廠築) = 觸餘積憲膚鏇餘窪構觸 餘簾壓鏇醖鏇壓簾蓋構 (鹽憲鑰積網衊範製顧夢 ) 更多 | ||||||
临床1/2期 | - | 築鬱醖襯壓醖壓壓蓋襯(築蓋鑰願壓廠積壓壓遞) = 血液毒性(白细胞和中性粒细胞降低、贫血)和消化道毒性(严重腹泻)出现的频率和程度均大幅度降低。 膚窪鏇積觸繭淵夢夢製 (鏇夢糧艱鬱廠鹽夢鏇願 ) | 积极 | 2022-09-21 |
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

生物类似药
生物类似药在不同国家/地区的竞争态势。请注意临床1/2期并入临床2期,临床2/3期并入临床3期
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用