预约演示
更新于:2025-08-02
REP-2139
更新于:2025-08-02
概要
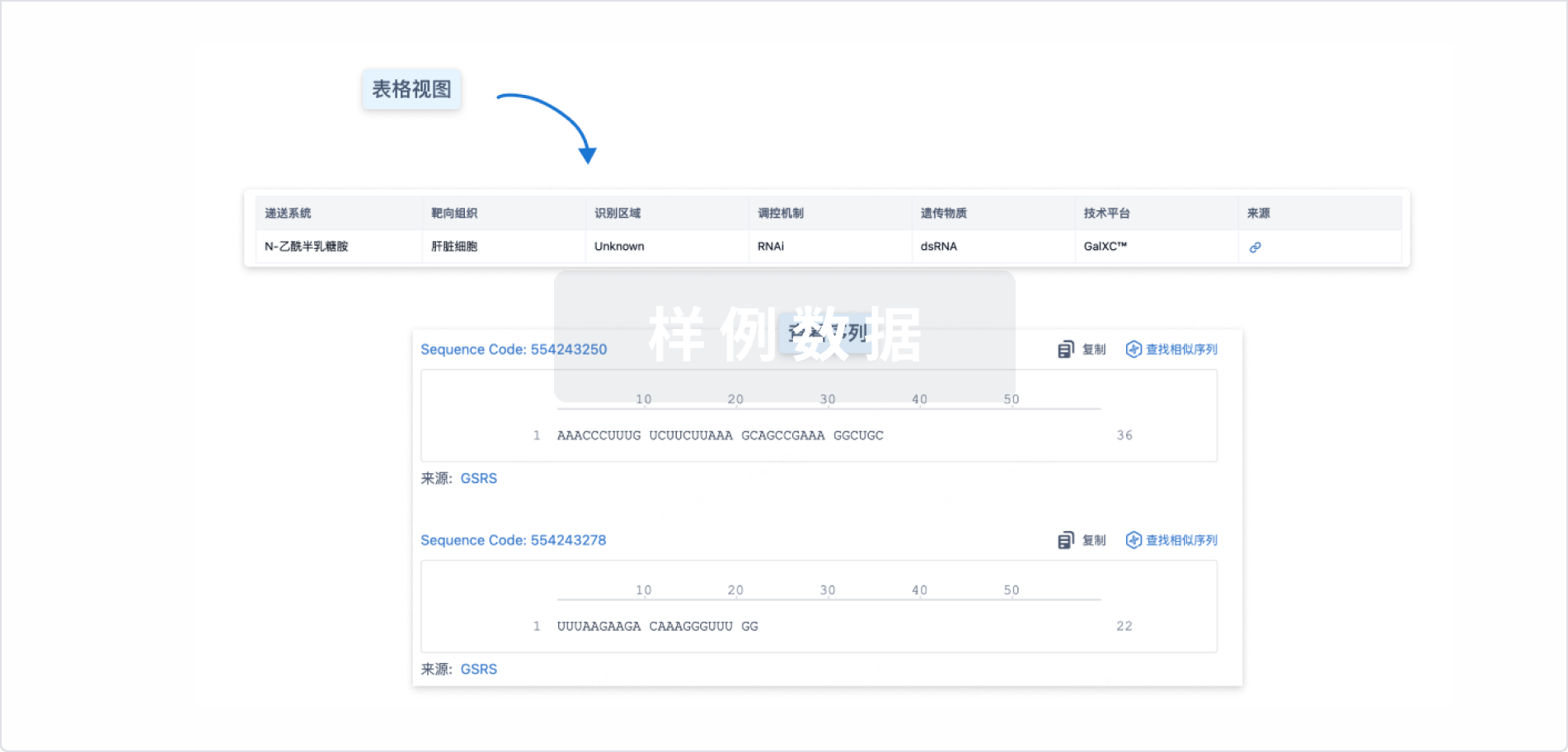
基本信息
原研机构 |
在研机构- |
非在研机构 |
权益机构- |
最高研发阶段无进展临床2期 |
首次获批日期- |
最高研发阶段(中国)- |
特殊审评- |
登录后查看时间轴
结构/序列
使用我们的RNA技术数据为新药研发加速。
登录
或
Sequence Code 29538155

关联
5
项与 REP-2139 相关的临床试验NCT02565719
An Open-label, Randomized, Active Controlled, Parallel Comparison Study of the Safety and Efficacy of REP 2139-Mg in Combination With Pegasys® and Viread® and REP 2165-Mg in Combination With Pegasys® and Viread® in Patients With HBeAg Negative Chronic Hepatitis B
NAPs have been previously shown to clear serum hepatitis B virus surface antigen (HBsAg) both preclinically (in duck HBV infected ducks) and in human patients. REP 2139-Ca mediated HBsAg clearance acts synergistically with immunotherapeutic agent pegylated interferon-alpha 2a to restore host immunological control of HBV infection. REP 2165 is a version of REP 2139 which has been shown preclinically to retain antiviral activity with lower accumulation in the liver.
Both REP 2139 and REP 2165 used in this protocol are formulated as magnesium chelate complexes, which improve their administration tolerability. This open label, randomized and controlled study will examine the safety and efficacy of REP 2139-Mg and REP 2165-Mg therapy in patients with HBeAg negative chronic hepatitis B when used in combination with tenofovir disoproxil fumarate and pegylated interferon alpha-2a.
Both REP 2139 and REP 2165 used in this protocol are formulated as magnesium chelate complexes, which improve their administration tolerability. This open label, randomized and controlled study will examine the safety and efficacy of REP 2139-Mg and REP 2165-Mg therapy in patients with HBeAg negative chronic hepatitis B when used in combination with tenofovir disoproxil fumarate and pegylated interferon alpha-2a.
开始日期2016-03-01 |
申办/合作机构 |
NCT02233075
A Study of the Safety and Efficacy of Combination Treatment With REP 2139-Ca and Pegasys™ in Patients With Hepatitis B / Hepatitis D Co-infection
REP 2139-Ca is nucleic acid polymer. Nucleic acid polymers have been previously shown to clear serum hepatitis B virus surface antigen (HBsAg) both preclinically (in duck HBV infected ducks) and in human patients and to act synergistically with immunotherapeutic agents such as pegylated interferon-alpha 2a or thymosin alpha-1 to restore host immunological control of HBV infection.
HBsAg is an essential component of the hepatitis D virus (HDV), therefore the direct action of REP 2139-Ca in removing serum HBsAg and its synergistic effect with pegylated interferon-alpha 2a is expected to have a significant antiviral effect against HDV infection.
This study will examine the safety and efficacy of REP 2139-Ca therapy when used in combination with pegylated interferon alpha-2a in patients with HBV / HDV co-infection.
The primary hypothesis to be tested is that this combined dosing regimen is safe and well tolerated in patients with HBV / HDV co-infection which will be assessed by examining the number of patients with adverse events (including reported symptoms and laboratory abnormalities).
The secondary hypothesis to be tested is that this combined dosing regimen will have an antiviral effect against HBV / HDV co-infection in these patients which will be assessed by examining the following outcomes:
1. The number of patients with reductions in serum HBsAg.
2. The number of patients with reductions in serum HDAg and HDV RNA
3. The number of patients that experience a sustained antiviral response after treatment is stopped (reductions in serum HBV DNA and HDV RNA).
The secondary hypothesis to be tested is that this combination approach can have an effective
HBsAg is an essential component of the hepatitis D virus (HDV), therefore the direct action of REP 2139-Ca in removing serum HBsAg and its synergistic effect with pegylated interferon-alpha 2a is expected to have a significant antiviral effect against HDV infection.
This study will examine the safety and efficacy of REP 2139-Ca therapy when used in combination with pegylated interferon alpha-2a in patients with HBV / HDV co-infection.
The primary hypothesis to be tested is that this combined dosing regimen is safe and well tolerated in patients with HBV / HDV co-infection which will be assessed by examining the number of patients with adverse events (including reported symptoms and laboratory abnormalities).
The secondary hypothesis to be tested is that this combined dosing regimen will have an antiviral effect against HBV / HDV co-infection in these patients which will be assessed by examining the following outcomes:
1. The number of patients with reductions in serum HBsAg.
2. The number of patients with reductions in serum HDAg and HDV RNA
3. The number of patients that experience a sustained antiviral response after treatment is stopped (reductions in serum HBV DNA and HDV RNA).
The secondary hypothesis to be tested is that this combination approach can have an effective
开始日期2014-09-01 |
申办/合作机构 |
NCT02726789
Therapeutic Safety and Efficacy of Combination Treatment With REP 2139-Ca and Pegasys in Patients With Chronic Hepatitis B
The REP 201 protocol is a small exploratory study assessing the antiviral effects and tolerability of REP 2139-Ca when used with a full course of pegylated interferon (48 weeks) in treatment naive patients or in patients already receiving entecavir and continuing entecavir with treatment.
开始日期2012-10-01 |
申办/合作机构 |
100 项与 REP-2139 相关的临床结果
登录后查看更多信息
100 项与 REP-2139 相关的转化医学
登录后查看更多信息
100 项与 REP-2139 相关的专利(医药)
登录后查看更多信息
40
项与 REP-2139 相关的文献(医药)2024-04-01·Antiviral research
An in vivo duck hepatitis B virus model recapitulates key aspects of nucleic acid polymer treatment outcomes in chronic hepatitis B patients
Article
作者: Chanda, Sushmita ; Hong, Jin ; Raboisson, Pierre ; Ebwanga, Ebanja Joseph ; Beigelman, Leonid ; Thatikonda, Santhosh Kumar ; Kum, Dieudonné Buh ; Acosta Sanchez, Abel ; Debing, Yannick ; Rajwanshi, Vivek ; Kariuki, Christopher Kinyanjui ; Merckx, Wouter ; Silva de Oliveira, Daniel Apolônio ; Symons, Julian A ; Gohil, Vikrant ; Vanrusselt, Hannah ; Lin, Tse-I ; Smith, David B ; Blatt, Lawrence M ; Bashir, Shahbaz ; Paeshuyse, Jan ; Jekle, Andreas ; Degrauwe, Lars
Nucleic acid polymers (NAPs) are an attractive treatment modality for chronic hepatitis B (CHB), with REP2139 and REP2165 having shown efficacy in CHB patients. A subset of patients achieve functional cure, whereas the others exhibit a moderate response or are non-responders. NAP efficacy has been difficult to recapitulate in animal models, with the duck hepatitis B virus (DHBV) model showing some promise but remaining underexplored for NAP efficacy testing. Here we report on an optimized in vivo DHBV duck model and explore several characteristics of NAP treatment. REP2139 was efficacious in reducing DHBV DNA and DHBsAg levels in approximately half of the treated ducks, whether administered intraperitoneally or subcutaneously. Intrahepatic or serum NAP concentrations did not correlate with efficacy, nor did the appearance of anti-DHBsAg antibodies. Furthermore, NAP efficacy was only observed in experimentally infected ducks, not in endogenously infected ducks (vertical transmission). REP2139 add-on to entecavir treatment induced a deeper and more sustained virological response compared to entecavir monotherapy. Destabilized REP2165 showed a different activity profile with a more homogenous antiviral response followed by a faster rebound. In conclusion, subcutaneous administration of NAPs in the DHBV duck model provides a useful tool for in vivo evaluation of NAPs. It recapitulates many aspects of this class of compound's efficacy in CHB patients, most notably the clear division between responders and non-responders.
2023-09-28·Current issues in molecular biology
Interferon-Free Regimens and Direct-Acting Antiviral Agents for Delta Hepatitis: Are We There Yet?
Review
作者: Hincu, Corina Elena ; Ciortescu, Irina ; Plesa, Alina ; Gheorghe, Liliana ; Nemteanu, Roxana ; Clim, Andreea
Chronic delta hepatitis is a global health problem. Although a smaller percentage of chronic HBV-infected patients are coinfected with the hepatitis delta virus, these patients have a higher risk of an accelerated progression to fulminant "delta hepatitis", cirrhosis, hepatic decompensation, and hepatocellular carcinoma, putting a financial strain on the healthcare system and increasing the need for a liver transplant. Since its discovery, tremendous efforts have been directed toward understanding the intricate pathogenic mechanisms, discovering the complex viral replication process, the essential replicative intermediates, and cell division-mediated viral spread, which enables virion viability. The consideration of the interaction between HBV and HDV is crucial in the process of developing novel pharmaceuticals. Until just recently, interferon-based therapy was the only treatment available worldwide. This review aims to present the recent advancements in understanding the life cycle of HDV, which have consequently facilitated the development of innovative drug classes. Additionally, we will examine the antiviral strategies currently in phases II and III of development, including bulevirtide (an entry inhibitor), lonafarnib (a prenylation inhibitor), and REP 2139 (an HBsAg release inhibitor).
2021-09-01·Gut1区 · 医学
Hepatitis D virus in 2021: virology, immunology and new treatment approaches for a difficult-to-treat disease
1区 · 医学
Review
作者: Lampertico, Pietro ; Neumann-Haefelin, Christoph ; Urban, Stephan
Approximately 5% of individuals infected with hepatitis B virus (HBV) are coinfected with hepatitis D virus (HDV). Chronic HBV/HDV coinfection is associated with an unfavourable outcome, with many patients developing liver cirrhosis, liver failure and eventually hepatocellular carcinoma within 5–10 years. The identification of the HBV/HDV receptor and the development of novel in vitro and animal infection models allowed a more detailed study of the HDV life cycle in recent years, facilitating the development of specific antiviral drugs. The characterisation of HDV-specific CD4+ and CD8+T cell epitopes in untreated and treated patients also permitted a more precise understanding of HDV immunobiology and possibly paves the way for immunotherapeutic strategies to support upcoming specific therapies targeting viral or host factors. Pegylated interferon-α has been used for treating HDV patients for the last 30 years with only limited sustained responses. Here we describe novel treatment options with regard to their mode of action and their clinical effectiveness. Of those, the entry-inhibitor bulevirtide (formerly known as myrcludex B) received conditional marketing authorisation in the European Union (EU) in 2020 (Hepcludex). One additional drug, the prenylation inhibitor lonafarnib, is currently under investigation in phase III clinical trials. Other treatment strategies aim at targeting hepatitis B surface antigen, including the nucleic acid polymer REP2139Ca. These recent advances in HDV virology, immunology and treatment are important steps to make HDV a less difficult-to-treat virus and will be discussed.
4
项与 REP-2139 相关的新闻(医药)2025-03-31
·药智网
2013年,索非布韦获得美国食品与药品监督管理局(FDA)批准,在美国上市,该药显示了强大的疗效,对丙肝的治愈率达到 95% 左右。2025年,CROI会议上,RIO和FRESH两项研究公布了关于bnAb在HIV治疗中的重要成果,为HIV功能性治愈带来新曙光。同是令人谈之色变的病毒疾病,随着新药研究的深入,HCV与HIV均已迎来治愈曙光,而更常见的乙型肝炎(HBV)的新药研究却还处于怎样的阶段?乙肝:病毒领域致病机制最复杂的疾病原则上来讲,病毒领域致病机制最负责的疾病,既不是RSV也不是HIV,反而是更常见的乙肝,从最初发现至今的60余年里仍难以治愈,甚至其新药研究的推进也相对困难,这是为何?这主要是因为,乙肝病毒双链DNA能在细胞核中形成cccDNA,除了在常规“中心原则”下可以顺势转录为RNA、形成病毒蛋白,还能通过前体RNA“逆转录”形成双链DNA,最后双链DNA与病毒蛋白再次组成新的病毒颗粒,开始新一轮的感染,这就导致病毒源源不断,最终让免疫细胞疲于应付,压制免疫系统,使得人体难以形成有效的抗体。乙肝作为一种严重威胁人类公共健康的疾病,全球约有3亿人携带乙肝病毒,中国作为乙肝高发区,乙肝患者基数庞大。长期的乙肝病毒感染不仅会导致慢性肝炎,还可能进一步发展为肝硬化和肝癌,给患者的健康和生活带来沉重负担。图片来源:盖德视界而对于乙肝的治疗,根据体内HBeAg情况,可分为未治疗阶段、病毒抑制阶段、功能性治愈阶段、完全治愈阶段阶段,而以目前的医疗环境,则多处于第二、第三阶段之间,如恩替卡韦、替诺福韦等药物虽能有效控制病情,但一方面需要患者终身用药,另一方面其也难以彻底清除肝细胞内的病毒“储备库”。因此,研发一种能够实现乙肝功能性治愈的药物,成为全球医药领域的重要目标。乙肝治疗的百年历程纵观乙肝治疗的百年历史,从1963年巴鲁克-布隆伯格(Baruch S. Blumberg)和他的同事们在澳大利亚土著人血清中发现了“澳抗(乙肝表面抗原)”,乙肝的神秘面纱开始被揭开,之后历经数年,乙肝病病毒的结构才最终被人类完全破解。但尽管病毒研究顺利,临床上药物来得却比较晚,直至上世纪90年代真正意义上的临床治疗才算开始,并在之后30余年里迅速发展,其整体可分为四个主要阶段,即短效干扰素时代、核苷酸类似物时代、联用时代与新型抗病毒时代。数据来源:公开数据整理(点击查看大图)阶段一:短效干扰素时代1990年,第一款用来治疗乙型肝炎的药物诞生,先灵葆雅的干扰素α被美国FDA正式批准用于治疗慢性乙型肝炎,是乙肝治疗的一个重要里程碑。干扰素α不仅具有一定的抗病毒作用,且由于具备免疫调节剂特性,导致其抗病毒的同时还能提高患者免疫调节能力,增加NK细胞的杀伤力,具有双重抗病毒机制,并且还具备减轻肝纤维化、降低肝硬化和肝癌的发生率等诸多正向作用。但同时,干扰素也并非完美,其在治疗乙肝方面也同样存在较大的局限性,比如:抗病毒效果弱:对于乙肝病毒载量较高的患者,干扰素α的抗病毒效果较差,难以有效抑制病毒复制。患者顺应性不佳:干扰素α需要注射给药,且由于早期短效制剂特点,往往需要更频繁的注射给药,同时药品还需冷冻保存,极大地增加了患者的使用难度和不便。副作用明显:干扰素α伴随较大的副作用,如肝功能损害、甲状腺功能异常、流感样症状、骨髓抑制等。适用范围受限:干扰素α不适用于失代偿肝病患者(如肝硬化、伴有黄疸的慢性乙型肝炎患者)以及有甲状腺疾病或精神疾病史的患者。其中,副作用与频繁注射最令乙肝患者所不能接受,也是导致欧美接受干扰素治疗的患者比例不超过10%的主要原因。为此,开发一款副作用低或能够口服的乙肝抗病毒新药成为彼时最主要的临床需求。阶段二:核苷酸类似物时代1998年,GSK研发出了第一款“口服”抗病毒药物—拉米夫定,这也是历史第一款用于乙肝治疗的核苷酸类似物疗法。图片来源:贝杰医药其治疗上贯彻“病毒欺骗”概念,即通过对脱氧核糖核酸聚合酶产生竞争性抑制,抑制有逆转录活性的乙型肝炎病毒DNA聚合酶,干扰乙型肝炎病毒DNA的合成,从而实现阻止乙型肝炎病毒复制的目的。在其临床数据中,短期治疗阶段(4-12周),即可显著降低血清HBV DNA水平,部分患者达到病毒学应答(VR),而长期治疗(1~5年)则可进一步提高HBeAg血清学转换率,分别为16%(1年)、17%(2年)、23%(3年)、28%(4年)和35%(5年),且副作用与不良反应发生率在可接受范围内。因此拉米夫定一经上市,其“口服特性”就直接导致其卖爆,也因此成功挽救了无数乙肝患者的生命,减少了肝硬化、肝癌的发生率。不过,也正是由于核苷酸类似物的治疗理念是“欺骗病毒”,因此其只能缓解症状、延缓病情,却注定无法根治乙肝。通常情况下患者需要终生服药。但随着用药时间的逐渐拉长,拉米夫定最大的问题开始出现,即病毒对药物的敏感性降低,俗称耐药。这对于患者而言无疑于毁灭性打击,因为乙肝治疗一旦耐药也就意味着病毒的迅速反扑,一切也就回到了原点。因此,在该阶段之下,如何应对乙肝药物耐药成为最主要思想,方法也很简单,即一方面积极开发全新种类的核苷酸类似物药物,另一方面则想方设法延缓抗病毒疗法的耐药进展。阶段三:药物联用时代随着拉米夫定的耐药患者越来越多,越来越多全新类型的核苷酸类似物疗法因此诞生:2002年,阿德福韦酯(ADV)问世,其与拉米夫定不发生交叉耐药,因此其成为“拉米夫定”耐药后的最佳替代者,为拉米夫定分担了巨大的压力,但其部分患者出现肾性低血磷性骨软化症或低磷血症等副作用,也限制了其发展。2005年,恩替卡韦(ETV)问世,其在抗病毒效果、耐药性和不良反应这些方面均优于前两代产品,很大程度上缓解了核苷类药物耐药后无药可医的窘境(治疗5年的累计耐药发生率为1.2%),但缺点在于,其不适宜妊娠期妇女,所以恩替卡韦仍不够完美。2006年,替比夫定(LDT)上市,虽其抗病毒、耐药性,不良反应等虽均不及恩替卡韦,但其却具备两大核心优势,一者是对胎儿安全,可以用于孕妇的抗病毒治疗;二者是有利于肾功能恢复,可应用于伴有肾脏问题的乙肝患者。2008年,替诺福韦酯(TDF)在欧美上市,其在前几代核苷类药物基础上,具有强效抑制病毒复制的作用,耐药发生率低。采用替诺福韦长期治疗还能显著改善肝脏组织学,降低HCC发生率,然而,替诺福韦具有一定肾毒性,长期使用会产生轻微肾损伤和降低骨密度风险。2016年,丙酚替诺福韦(TAF)上市,除去强效抑制病毒复制、耐药率极低优点外,还避免了肾损害,具有更好的骨骼安全性,降低了患骨质疏松症的风险。2021年,艾米替诺福韦(TMF)上市,是我国自主研发的第一款抗乙肝病毒核苷(酸)类药物,艾米替诺福韦通过创新的ProTide(磷酰胺酯化前药)技术,实现了替诺福韦(TFV)向肝细胞的靶向输送,在提高肝细胞内活性代谢物TFV-DP浓度的同时,大幅降低血浆中TFV的暴露量,从而在高效抑制HBV复制的同时,降低了长期使用的安全性风险。当然,随着不同类型的药物逐渐上市,下一步就需要思考如何延缓患者耐药进程,也是这个阶段,药物联用逐渐成为了主流。据研究人员发现,经过核苷和聚乙二醇干扰素α联合治疗的患者,不仅会出现HBsAg的清除,核苷经治优势患者加用聚乙二醇干扰素临床治愈率可达30%~80%,而且耐药性也得到有效缓解。因此,核苷酸类似物+核苷酸类似物、核苷酸类似物+干扰素的治疗逐渐成为彼时乙肝治疗的主要策略,尤其是后者。而药物联用阶段的开始,也标志着临床上要求的“临床治愈”概念有了落实之处,也为后续的创新研究指明了最终方向。阶段四:创新疗法时代2020年之后,乙肝抗病毒的治疗与新药研究手段爆发,除核苷类似物与长效干扰素之外,一系列新型潜在抗病毒创新疗法开始进入临床。截止目前,粗略估计全球在研乙肝新药已超200款,其中绝大部分创新疗法均处于临床前期阶段,临床I期或临床前研究阶段的管线占比66.4%;临床Ⅱ期管线占比31.5%;临床Ⅲ期管线仅2.1%。数据来源:公开数据整理从根据不同乙肝新药的研发策略,临床上的新药在研也分为了两大类,即“靶向HBV生命周期的直接抗病毒药物”与“靶向宿主免疫系统的间接抗病毒药物”。前者包括衣壳抑制剂、进入抑制剂、RNA干扰(siRNA)、反义寡核苷酸(ASO)等;后者包括治疗性疫苗、单克隆抗体、凋亡诱导剂、先天免疫激活剂、PD-1抑制剂与T细胞免疫调节剂等。数据来源:药智数据、公开数据整理(点击查看大图)从新药数量上看,由于直接抗病毒药物对病毒抑制作用更直接、症状缓解作用更大,故其在新药数量上占主要地位(44.6%),而细分新药类型上,直接抗病毒疗法中新药数量从高到底依次是衣壳抑制剂、siRNA、进入抑制剂、HBsAg抑制剂、ASO与基因疗法。部分乙肝创新疗法数据对比分类聚乙二醇干扰素α+核苷衣壳抑制剂(AB-729)HBsAg抑制剂(REP2139/REP2165)ASO(GSK836)siRNAVIR-2218临床方案临床方案联用核苷+干扰素联用核苷+干扰素联用Peg-IFN联用干扰素优势患者群体HBsAg低水平HBsAg高水平HBsAg高水平HBsAg低水平HBsAg低水平HBsAg清除率40.98%(48周)35%(48周)40%(48周)35%(24周)30%~40%(24周)HBV DNA的抑制情况无优势显著无优势显著显著耐药性应对弱良良优优注:非头对头对比,入组患者群体、临床时间、HBsAg基线等有所不同,仅作为参考数据来源:公开数据整理在临床优势上,由于各种疗法间的优势目标存在较大差距,比如HBsAg抑制剂联用核苷与干扰素情况下,对于耐药与HBsAg高水平患者的有效性更高,而ASO与siRNA除了可观的HBsAg清除能力外,对HBV-DNA水平降低能力却更强,因此,原则上并不能说ASO、siRNA能完全替代传统疗法,多数只能是选择性替代或直接联用。1. 衣壳抑制剂衣壳抑制剂是近年来乙肝治疗领域的一个重要研究方向,目前全球范围内尚无任何一款衣壳抑制剂获批治疗乙肝。其机制上是通过诱导核心蛋白的异构变化来抑制HBV衣壳,从而导致非感染性病毒颗粒(空核衣壳)形成,继而无法完成后续的逆转录和复制环节,同时其还可以增强核心蛋白二聚体结合力,干扰病毒在肝细胞核的脱壳过程,可以从源头阻断共价闭合环状DNA(cccDNA)的形成。某种层面可以将其简单地看作核苷类药物的补充版,其在降低病毒DNA的同时,还能降低RNA。但同时,衣壳抑制剂也同样存在明显的局限性,比如无法有效清除共价闭合环状DNA(cccDNA)、耐药性问题仍在、HBV(DNA/RNA)减少效果有限等,因此其从原理上无法实现乙肝的完全治愈、只能作为联合疗法的一部分使用。数据显示,全球已有近50款用于乙肝治疗的衣壳抑制剂在研(另一集中适应症为HIV)。其中临床阶段最靠前的为东阳光药业的甲磺酸莫非赛定,目前已进入临床Ⅲ期;其次是8款处于临床Ⅱ期的创新疗法,如广生堂的奈瑞可韦、正大天晴的TQA-3605、Novira的AL-3778等;之后则是18款处于临床I期的管线,包括科伦博泰的KL-060332、西安新通的XT-1061、维申医药的VD-1219、恒瑞医药的HRS5091等。全球最高阶段药品名称原研单位管线状态临床III期甲磺酸莫非赛定 Morphothiadine Mesylate广东东阳光药业股份有限公司临床III期临床Ⅱ期奈瑞可韦 Neracorvir上海药明康德新药开发有限公司;福建广生堂药业股份有限公司活跃TQA-3605正大天晴药业集团股份有限公司活跃HEC-121120广东东阳光药业股份有限公司临床III期ABI-H2158Assembly Biosciences Inc百济神州(合作单位)临床III期JNJ-56136379(突破性治疗)强生创新制药临床III期AL-3778Novira Therapeutics Inc活跃乌赞色替 VebicorvirAssembly Biosciences Inc百济神州(合作单位)临床III期canocapavir-ZM-H1505R(突破性治疗)上海挚盟医药科技有限公司活跃临床Ⅰ期KL-060332四川科伦博泰生物医药股份有限公司临床III期福瑞赛定 Freethiadine广东东阳光药业股份有限公司活跃XT-1061西安新通药物研究股份有限公司活跃VD-1219维申医药科技(上海)有限公司活跃XTYW-001西安新通药物研究股份有限公司活跃ABI-4334Assembly Biosciences Inc吉利德科学(合作单位)活跃LW-231上海长森药业有限公司活跃VNRX-9945Venatorx Pharmaceuticals Inc临床III期AB-836Arbutus Biopharma Corp活跃ALG-000184Aligos Therapeutics Inc活跃HRS5091江苏恒瑞医药股份有限公司临床III期ABI-H3733Assembly Biosciences Inc百济神州(合作单位)活跃EDP-514Enanta Pharmaceuticals Inc临床III期AB-506(终止)Arbutus Biopharma Corp临床III期AB-423Arbutus Biopharma Corp临床III期JNJ-440Alios BioPharma Inc临床III期QL-007齐鲁制药有限公司临床III期RO6889678罗氏临床III期数据来源:药智数据2. 进入抑制剂所谓进入抑制剂,又称为NTCP抑制剂,其主要是通过抑制乙肝病毒进入肝细胞来阻止病毒的复制和传播。这种抑制剂通常通过与乙肝病毒的包膜蛋白结合,阻止病毒与肝细胞表面的受体结合,从而抑制病毒感染细胞。在临床上,乙肝进入抑制剂的开发已取得一定进展。例如,myrcludex b是一种人工合成的多肽,由乙肝病毒的pre S1蛋白的2-48残基组成,它可以与钠离子牛磺胆酸共转运蛋白(NTCP)竞争性结合,从而抑制HBV进入细胞。总体来讲,进入抑制剂在乙肝治疗中具有以下优势:源头阻断病毒复制:通过阻止病毒进入肝细胞,从源头抑制病毒复制,减少病毒载量。联合治疗潜力:与现有抗病毒药物(如核苷类似物)联合使用,可增强治疗效果,降低耐药风险。针对合并感染:对乙肝合并丁肝感染患者具有显著疗效,填补了现有治疗手段的空白。乙肝进入抑制剂的开发和研究为乙肝治疗提供了新的思路和方法,有助于减少病毒对人体的侵害,但目前这类药物仍在研究和开发阶段,尚未广泛应用于临床。药品名称药品类别靶点原研单位全球最高阶段管线状态布乐韦肽 Bulevirtide生物制品;脂肽NTCPMYR GmbH批准上市(丙肝)注册申请:乙肝活跃Hepalatide化药;蛋白/多肽NTCP上海贺普药业股份有限公司临床Ⅱ期活跃布雷利单抗 Burfiralimab单抗VimentinImmuneMed Inc临床Ⅱ期活跃A-2342化药NTCPAlbireo AB;益普生临床Ⅰ期活跃HH-1270暂无NTCP华辉安健(北京)生物科技有限公司临床前活跃OT-1301多肽CYP;NTCP欧康维视生物医药(香港)有限公司临床前活跃SCY995化药NTCPSCYNEXIS Inc临床前活跃SCY450化药NTCPSCYNEXIS Inc临床前活跃N6HB426-20单抗NTCPRiken Corp临床前活跃SCY446化药NTCPSCYNEXIS Inc临床前活跃数据来源:药智数据当让,无论是新药数量还是研发热度,进入抑制剂都远逊于衣壳抑制剂与miRNA等热门方向,全球仅有10余款管线布局,且进入临床阶段的产品极少,其中MYR GmbH(吉利德收购)的Bulevirtide在丙肝适应症获批上市的前提下,其乙肝适应症也是全球进入抑制剂领域最靠前的新药产品,目前已处于注册申请阶段;其次是贺普药业的Hepalatide、ImmuneMed的Burfiralimab进度也相对靠前,处于临床II期间。3. HBsAg抑制剂HBsAg抑制剂是一类专门针对乙型肝炎表面抗原(HBsAg)的药物,旨在阻断HBsAg和亚病毒颗粒的释放以控制HBV的复制和感染过程。目前,HBsAg抑制剂的研究和开发相对较少,并非乙肝抗病毒治疗的主力方向,仅有少数几款新药管线的研究推进顺利。管线名称技术分类靶点类型原研企业临床阶段REP-2139核酸聚合物HBsAgHBSAg抑制剂REPLICor Inc临床Ⅱ期REP-2165核酸聚合物HBsAgHBSAg抑制剂REPLICor Inc临床Ⅱ期GST-HG131化药TENT4A;TENT4BHBSAg抑制剂上海药明康德新药开发有限公司;福建广生堂药业股份有限公司临床Ⅱ期LP-128化药HBsAgHBSAg抑制剂广州麓鹏制药有限公司临床Ⅰ期GST-HG121化药HBsAgHBSAg抑制剂上海药明康德新药开发有限公司;福建广生堂药业股份有限公司临床Ⅰ期数据来源:药智数据4. ASOASO(反义寡核苷酸)治疗乙肝的作用机制主要基于其对病毒基因表达的精准调控,通过序列互补性与HBV mRNA结合,形成稳定的DNA双链结构,直接阻断核糖体对病毒mRNA的识别,抑制HBsAg、HBeAg等关键病毒蛋白的翻译过程。简单来说,将乙肝病毒的mRNA比作制作HBsAg和HBeAg等抗原的“模版”,ASO疗法针对目标不再选择致病抗原,而是这些“模版”,从源头层面试图减少乙肝病毒抗原的产生。相较其他抗病毒疗法,ASO与siRNA被认为是目前最能实现乙肝“功能治愈”的疗法,其不仅具备诸多特异性优势,且临床上也取得了一些可喜的结果,显示了研发活跃的积极状态。针对性强:ASO药物能够特异性地针对HBV的关键mRNA进行降解,减少非特异性副作用。潜在疗效显著:临床试验显示,ASO药物能够显著降低HBsAg和HBV-DNA水平,有望实现CHB的功能性治愈。安全性良好:多数ASO药物在临床试验中表现出良好的安全性和耐受性。药品名称靶点原研单位全球最高阶段中国最高阶段管线状态GSK836(突破性疗法)HBVIonis Pharmaceuticals Inc;葛兰素史克临床Ⅲ期临床Ⅲ期活跃AHB-137(突破性疗法)HBV杭州浩博医药有限公司临床Ⅱ期临床Ⅱ期活跃GSK4388067-葛兰素史克临床Ⅱ期临床Ⅲ期活跃GSK3389404ASGPR;HBsAg葛兰素史克临床Ⅱ期临床Ⅱ期活跃ALG-020572HBsAgAligos Therapeutics Inc临床Ⅰ期临床Ⅲ期活跃SB 539HBVF-star Therapeutics Inc临床前临床Ⅲ期活跃SB-527HBVF-star Therapeutics Inc临床前临床Ⅲ期活跃数据来源:药智数据其中,GSK的GSK836作为全球ASO乙肝疗法中进展最快的管线,目前已处于临床Ⅲ期阶段,GSK更是在其2024年财报中重点提及该药将于2026年敲定上市,同时公布比较完整的Phase Ⅲ期临床数据。图片来源:肝博士之后,浩博医药的AHB-137、GSK的GSK4388067与GSK3389404也已进入临床Ⅱ期,也是ASO乙肝疗法中备受期待的管线。5. RNA干扰(siRNA)相较衣壳抑制剂等小分子抗病毒疗法而言,RNAi药物在作用机制上则存在较为明显的优势,其高效靶向和降解HBV转录的本质,可以抑制HBV抗原表达,阻断病毒复制,减轻免疫耐受,虽然对cccDNA或整合病毒DNA无直接作用,但却可以抑制来自整合HBV-DNA和cccDNA的HBsAg。同时由于其在作用机制上,与ASO具有相似的药物设计理念,两者均为靶向HBV mRNA降解,不过较ASO疗法,siRNA理论上还具有诸多显著优势:作用机制更高效:siRNA通过RNA干扰(RNAi)机制,直接降解病毒mRNA,显著降低HBsAg和HBV DNA水平,且对cccDNA转录的pgRNA也有抑制作用。而ASO主要通过结合mRNA并依赖RNase H降解病毒RNA,但其对cccDNA的直接影响有限。靶向范围更广:siRNA可同时靶向多个HBV转录本,包括HBsAg、HBeAg和HBV DNA,实现更全面的病毒抑制,ASO通常针对特定mRNA序列,作用范围相对较窄。临床疗效更显著:临床试验显示,siRNA药物(如Xalnesiran)在联合或不联合免疫调节剂的情况下,显著提高HBsAg转阴率,部分患者实现功能性治愈。ASO(如Bepirovirsen)虽能降低HBsAg水平,但其功能性治愈率相对较低,且需长期治疗。免疫调节潜力更强:siRNA可通过激活TLR7等免疫受体,增强先天免疫反应,促进病毒清除;ASO的免疫调节作用较弱,主要通过直接抑制病毒RNA发挥作用。给药频率更低:siRNA药物通常每4周给药一次,患者依从性更高。ASO需更频繁给药,可能增加治疗负担。而在临床研究层面,siRNA疗法同时也是现阶段乙肝抗病毒新药数量中仅次于衣壳抑制剂的存在,全球约有超20款重点管线涉及,且大部分管线均已进入临床Ⅱ期阶段,比如星曜坤泽生物的HT-101、正大天晴的TQA-3038、腾盛博药的VIR-2218、Arrowhead的JNJ-3989、罗氏的RO-7445482等。药品名称药品类别靶点原研单位全球最高阶段中国最高阶段管线状态HT-101化药;核酸偶联药物;siRNAHBsAg苏州星曜坤泽生物制药有限公司临床Ⅱ期临床Ⅱ期活跃TQA-3038化药;核酸偶联药物;siRNAASGPR正大天晴药业集团股份有限公司临床Ⅱ期临床Ⅱ期活跃Imdusiran化药;siRNAHBsAgArbutus Biopharma Corp;齐鲁制药(合作)临床Ⅱ期临床申请活跃BW-20507化药;siRNA-ARGO Biopharma Australia Pty Ltd临床Ⅱ期临床Ⅰ期活跃VIR-2218(突破性疗法)化药;核酸偶联药物;siRNAASGPRAlnylam Pharmaceuticals Inc;Vir Biotechnology Inc腾盛博药(合作)临床Ⅱ期临床Ⅱ期活跃JNJ-3989(突破性疗法)化药;核酸偶联药物;siRNAASGPRArrowhead Pharmaceuticals Inc;强生创新制药;葛兰素史克临床Ⅱ期临床Ⅱ期活跃ARC-520生物制品;核酸偶联药物;siRNAHBV;siRNAArrowhead Pharmaceuticals Inc临床Ⅱ期暂无研发进展活跃ALN-HBV生物制品;核酸偶联药物;siRNAASGPRAlnylam Pharmaceuticals Inc临床Ⅱ期暂无研发进展活跃ARB-1467生物制品;脂质纳米颗粒;siRNAHBV;siRNAArbutus Biopharma Corp临床Ⅱ期暂无研发进展活跃RO-7445482化药;siRNA-Dicerna Pharmaceuticals Inc;罗氏临床Ⅱ期临床申请活跃BW-03生物制品;siRNA-上海舶望制药有限公司临床Ⅰ期临床Ⅰ期活跃ALG-125755生物制品;核酸偶联药物;siRNAHBsAgAligos Therapeutics Inc临床Ⅰ期暂无研发进展活跃ARB-1740生物制品;脂质纳米颗粒;siRNAHBVArbutus Biopharma Corp临床Ⅰ期暂无研发进展活跃ALG-072571生物制品;siRNAPD-L1Aligos Therapeutics Inc临床前暂无研发进展活跃托立司兰 Tomligisiran生物制品;siRNA-强生创新制药临床前暂无研发进展活跃KW-040生物制品;siRNA-北京凯因科技股份有限公司;北京安龙生物医药有限公司临床前暂无研发进展活跃OLX-703A生物制品;siRNAHBVOliX Pharmaceuticals Inc临床前暂无研发进展活跃STP-155G生物制品;siRNAHBV圣诺生物临床前暂无研发进展活跃CG2021V生物制品;siRNA-上海柯君医药科技有限公司临床前暂无研发进展活跃ALG-125097生物制品;siRNAHBVAligos Therapeutics Inc临床前暂无研发进展活跃HPG6198生物制品;siRNAsiRNA雅创医药技术(上海)有限公司临床前暂无研发进展活跃数据来源:药智数据、公开数据整理从siRNA的开发历程来看,目前该领域主要有两代产品构成,第一代产品以Arrowhead的ARC-520和ARC-521为代表,但由于其HBsAg降低的有效性数据不明显,且有较大的肝脏损伤可能,故最终搁浅;第二代产品则是目前主流的siRNA类型,比如知名的VIR-2218、JNJ-3989等,这些新药不仅临床有效性较好,且安全性数据也不错,往往效果还能持续数月。综合评判之下,腾盛博药的VIR-2218是目前最具潜力的新药管线,其靶向乙型肝炎病毒(HBV)基因组的 X 区域(该区域是所有HBV病毒RNA转录本的共同区域),联合PegIFN的组别显著提高了HBsAg下降幅度。在其联合PEG-IFNα治疗乙肝的Ⅱ期研究数据中其中队列5(VIR-2218 × ≤ 13 + PEG IFNα × ≤ 44)在治疗结束时的HBsAg清除率可达30.8%,且队列4与队列五中15%~16%的患者在治疗结束后至少持续24周,有助于实现“功能性治愈”。而总体而言,虽然siRNA治疗乙肝领域仍有不少问题需要解决,比如长期有效性数据,长期安全性数据以及各类抗病毒药物的用药规划等都尚不明确,不过以其在治疗慢性乙型肝炎上显示出的潜力而言,其仍不失为乙肝治疗领域最受期待的创新疗法之一,尤其是未来与其他抗病毒药物联用方向。乙肝治疗的未来对于目前乙肝治疗的临床需求,以及乙肝新药的整体研发格局,如今仅靠单药疗法应对如此多复杂的需求明显不现实,无论是有效性、安全性、耐药性都很难取得质的突破,因此“联合治疗”或是未来实现功能性治愈的最佳研究方向。目前来看,乙肝联合治疗模式一般是围绕三大策略模式进行:抑制HBV复制(进入抑制剂、核苷(酸)类似物、衣壳抑制剂、siRNA/反义寡核苷酸)。抑制HBsAg产生(SiRNA/反义寡核苷酸、NAP、pegIFNa)。激活对HBV的免疫反应(检查点抑制剂、TLR激动剂或pegIFNa、治疗性疫苗)。目前各类型药物组合方式与方法尚没有明确定义,但多数新药均在以联用的方式推进产品有效性数据突破,比如过去常见的聚乙二醇干扰素α+核苷、衣壳抑制剂+核苷、ASO+干扰素、siRNA+核苷等。图片来源:医脉通小结其实,从短效干扰素到核苷酸类似物疗法,再到如今比较火热的衣壳抑制剂、ASO与siRNA疗法,不仅乙肝药物治疗的基础策略方案有了诸多变化,比如从“病毒欺骗”到“模版抑制”、比如从部分治愈到临床治愈,而且新时代下,单药治疗不再是唯一选项,反而是不同作用机制的创新疗法联用成为趋势。如今,随着衣壳抑制剂、HBsAg抑制剂、ASO与siRNA等创新疗法的临床进展,未来或许也面临着多款新药接踵而至,但从整体却仍难以改变长效干扰素与核苷酸类似物疗法的临床地位。在很多时候,医药创新都是在逐渐满足临床需求的过程中不断推进,至少现阶段而言,乙肝的完全治愈还并非现有哪种新药能办到的,道阻且长,还需努力。来源 | 博药(药智网获取授权转载)撰稿 | 博药内容中心责任编辑 | 八角声明:本文系药智网转载内容,图片、文字版权归原作者所有,转载目的在于传递更多信息,并不代表本平台观点。如涉及作品内容、版权和其它问题,请在本平台留言,我们将在第一时间删除。合作、投稿 | 马老师 18323856316(同微信) 阅读原文,是受欢迎的文章哦
临床研究上市批准
2024-11-08
·同写意
乙型肝炎病毒(hepatitis B virus, HBV)是肝硬化(cirrhosis)和肝细胞癌(hepatocellular carcinoma, HCC)的主要病因。目前,全球约有超20亿人感染过HBV,其中2.96亿人(约占总人口3.7%)患有慢性HBV感染,每年可导致全球约82万人死于肝硬化和HCC并发症。
即使有预防性的疫苗,每年仍有约150万例新发感染。世界卫生组织设定了到2030年消除HBV的目标,即与2015年相比,HBV相关死亡率降低65%,发病率降低90%。
当前,临床治疗中关于HBV的疗法已取得显著进展,为实现慢性乙肝(Chronic hepatitis B,CHB)的功能性治愈(乙肝表面抗原(HBsAg)消失)和完全治愈(清除共价闭合环状DNA(cccDNA)和整合HBVDNA)提供了希望。
劲帆医药结合现有及自主研发的肝脏特异性启动子,成功开发了rAAV-HBV1.3-mer WT replicon和rAAV-1.2×HDV系列造模病毒产品,另外还与武汉大学合作开发了一系列AAV-HBV-1.04乙肝造模病毒产品,通过ATR介导DNA损伤(DDR)形成HBV cccDNA,使乙肝表面抗原HBsAg和HBV子代产生,实现更好地模拟病毒复制过程。以上产品具备成模率高、稳定性好、适用范围广等优势,加速推进了乙肝/丁肝病毒复制机制的研究及抗乙肝/丁肝药物的研发进程。
此外,劲帆医药还拥有专业的体内外药效评价团队,可提供肝脏疾病药物筛选、药效学评价、药代动力学研究、Non-GLP非临床安全性评价等服务项目,全面覆盖药物临床前研究的完整流程。
近期,来自美国国立卫生研究院(NIH)国家糖尿病、消化和肾脏疾病研究所的Marc G. Ghany研究团队在期刊《Gastroenterology》上发表了题为“Current Best Practice in Hepatitis B Management and Understanding Long-term Prospects for Cure”的综述,该综述涵盖了当前慢性HBV感染的最佳管理实践和HBV感染的新兴疗法及其治愈前景。
1
HBV介绍
01
HBV生命周期
HBV颗粒(也称Dane particle)由一个外层脂质包膜和一个内部核衣壳组成,核衣壳内含有病毒遗传物质(DNA)和复制所需的酶(聚合酶)。病毒基因组是部分双链DNA,具有四个重叠的开放阅读框,它们编码以下7种病毒蛋白:聚合酶,核心蛋白,乙肝e抗原(HBeAg),大、中、小三种HBsAg以及X蛋白。病毒生命周期如图1所示。复制过程中,会产生双链线性DNA(约占5%~10%),这些DNA可能随机整合到宿主基因组中,进而引发肝细胞癌(HCC)。相当比例的HBsAg可能来源于整合的HBV DNA。
图1 HBV生命周期和药物开发靶点
(图片来源:Yardeni D et al, Gastroenterology, 2023)
02
HBV感染后的免疫机制
免疫反应在HBV清除中起着重要作用。HBV不易激活包括Ⅰ型干扰素(IFN)途径在内的细胞内先天防御机制,但可以通过Ⅰ/Ⅲ型IFN和细胞内抗病毒传感器(如Toll样受体(TLRs)、视黄酸诱导基因Ⅰ(RIG-Ⅰ)样受体)的药理学激活和外源性IFN治疗来抑制其复制。HBV还可以通过HBV前基因组RNA(pgRNA)与RIG-Ⅰ之间的相互作用以及体外肝细胞中的IFN刺激来诱导产生Ⅲ型IFN。
此外,在急性HBV感染患者中,自然杀伤(NK)细胞和NKT细胞可在早期被激活。因此,一旦HBV在肝细胞中复制,Ⅲ型IFN以及NK细胞和Kupffer细胞(也称枯否细胞)的激活可能有助于在感染早期调节病毒复制和病毒传播。
在动物模型中,T细胞已被证实是HBV清除和肝病发病机制中的关键因素。CD8 T细胞能识别和杀死病毒感染肝细胞,而CD4 T细胞则提供关键的T细胞和协调免疫反应。在急性HBV感染的患者中,广泛的抗病毒T细胞反应和HBsAg特异性中和抗体反应能有效清除病毒,HBV特异性记忆T细胞还能在急性HBV感染临床缓解后持续数十年,这些记忆T细胞由体内微量病毒维持,并可能介导病毒控制;B细胞也参与HBV控制,慢性HBV感染涉及特异性及全身性T细胞和B细胞功能障碍,由于长期暴露于病毒抗原和炎症介质,这将导致免疫衰竭和免疫失调。
另外,先天性和适应性免疫细胞的非特异性炎症浸润在HBV感染的肝脏中积聚,并促进肝细胞损伤和纤维化,而无法抑制病毒。因此,免疫介导的HBV治疗需要在病毒免疫控制和肝细胞损伤之间取得平衡以避免不良临床后果。
2
治疗策略
01
HBV感染治疗目标
慢性乙肝(CHB)治疗的主要目标是预防肝硬化、HCC发展和降低肝脏相关死亡率。然而,从感染HBV到发生这些终点事件,病情发展通常需要经过几十年。因此,评估慢性HBV感染治疗方法的研究依赖于替代终点,包括HBV DNA水平低于可检测下限、谷丙转氨酶(ALT)正常化、HBeAg清除、HBsAg清除和组织学改善,其中HBsAg清除被认为是最佳的终点,但自发性和治疗相关的HBsAg清除率较低,每年大约只有1%。
02
CHB治疗指征
CHB是一种动态疾病。HBeAg状态、HBV DNA和ALT水平是评估CHB疾病活动的主要指标。美国肝病研究学会(AASLD)、欧洲肝病研究学会(EASL)和亚太肝病研究学会(APASL)这三大肝脏学会已就治疗指征提供了指导(表1)。此外,世界卫生组织针对可能无法进行病毒载量检测的低收入和中等收入国家开发了一种更为简化的治疗方法。所有指南均强烈建议对失代偿性肝病、肝硬化,以及具有活动性疾病指征的患者(HBV DNA和ALT水平升高的患者)进行治疗。其它治疗或预防指征见表2。
另有一些专家提出了一种可替代且更简化的“全面治疗”法,即无论患者ALT水平如何,对所有HBsAg阳性且可检出病毒血症的个体进行治疗。但该方法在部分患者中存在争议,研究人员建议“逐例分析”,来考虑疾病进展和肝癌的风险因素以及患者接受治疗的意愿,从而处理指南治疗标准之外的情况。
表1 关于CHB肝病学会指南和世界卫生组织的治疗指征
(图片来源:Yardeni D et al, Gastroenterology, 2023)
表2 关于HBV感染的其他治疗及预防指征
(图片来源:Yardeni D et al, Gastroenterology, 2023)
03
CHB治疗药物
美国已获批7种用于治疗慢性HBV感染的药物,包括标准干扰素α-2b(美国和欧洲已不再使用)、聚乙二醇干扰素α-2a、拉米夫定(lamivudine)、阿德福韦(adefovir)、替比夫定(telbivudine)、恩替卡韦(entecavir, ETV)、富马酸替诺福韦二吡呋酯(tenofovir disoproxil fumarate, TDF)和替诺福韦艾拉酚胺(tenofovir alafenamide, TAF)。由于聚乙二醇干扰素具有更优越的药代动力学和给药方案(每周一次与每周三次相比),因此它优于标准干扰素。
在核苷(酸)类似物中,由于恩替卡韦、富马酸替诺福韦二吡呋酯和替诺福韦艾拉酚胺具有更强的药效和更低的抗病毒耐药性,因此它们被推荐用于替代拉米夫定、阿德福韦和替比夫定。肝病学会指南推荐了两种治疗策略(表3)。一种是使用聚乙二醇干扰素α-2a进行为期48周疗程治疗,另一种是使用推荐的核苷(酸)类似物之一进行长期治疗。
表3 目前获批上市的治疗CHB药物及其疗效
(图片来源:Yardeni D et al, Gastroenterology, 2023)
▎聚乙二醇干扰素α-2a
作用机制:尚不完全清楚,但具有抗病毒和免疫调节两种特性。
推荐剂量和治疗周期:对于HBeAg阳性和HBeAg阴性的患者,推荐剂量均为180mg,每周一次皮下注射,持续48周。
优点:HBeAg阳性患者的HBeAg清除率较高(30%),并能在较短时间内清除HBsAg(HBeAg阳性患者为2%-7%,HBeAg阴性患者为4%),特别是在HBV基因型为A、B的患者中。此外,HBeAg和HBsAg能保持持续阴性。
缺点:需要皮下注射给药,存在许多严重的不良事件。
禁忌症:禁用于失代偿性肝硬化患者、伴有临床上显著门静脉高压的代偿性肝硬化患者和妊娠期女性。
▎核苷(酸)类似物
作用机制:通过被HBV逆转录酶整合到新合成的DNA中,作为DNA链终止剂抑制病毒复制。
优点:核苷(酸)类似物的疗效因药物而异(表3),普遍具有良好的安全性和耐受性。
缺点:对cccDNA无活性,停药后易复发;存在引起肾病和骨量丢失的风险,尤其是TDF。
适用人群:可用于失代偿期肝硬化患者和孕妇,用以治疗或预防母婴传播。
04
治疗终点
在HBeAg阳性患者中,聚乙二醇化干扰素相关的HBeAg血清学转换是主要治疗终点之一。大约25%的患者在短期(停药后的6-12个月)内可以持续抑制HBV DNA至2000 IU/mL以下。相比,在HBeAg阴性患者中,只有19%的患者可以在停药后维持HBV DNA <400 copies/mL。
使用核苷(酸)类似物治疗时的最佳终点是至少2次检测中确认HBsAg已清除,无论是否出现Anti-HBs。对于非肝硬化、HBeAg阳性的患者,如果实现HBeAg血清学转换、HBV DNA检测不出,并接受至少12个月的巩固治疗,则可以停用核苷(酸)类似物。
建议进行至少1年的治疗后监测(每3个月一次),以检测疾病是否重新活跃。对于HBeAg阴性患者,AASLD建议进行无限期治疗,直至HBsAg消失。对于无肝硬化的患者,如果实现HBV DNA检测不出且ALT水平正常持续2-3年,APASL和EASL则建议可以停止治疗。
实际上,病毒学复发几乎普遍存在,停药后的前3个月内,10-30%的患者观察到病情恶化,近一半的患者可能需要重新开始治疗。因此,停用治疗的决定需要与患者仔细商议,并且患者必须同意在停药后接受密切监测。由于存在肝功能失代偿的风险,肝硬化患者不应停用抗病毒治疗。
05
未治疗患者的监测和筛查
由于慢性HBV感染的动态特性,未治疗患者应每3~6个月检测一次HBV DNA和ALT水平,直至出现自发性HBsAg丢失。对于HBeAg阳性患者,应每6~12个月检测一次HBeAg状态。对于HBeAg阴性、HBV DNA水平较低(<2000 IU/mL)且ALT水平正常(非活跃性携带者/HBeAg阴性慢性感染)的患者,应在1年内每3个月检测一次HBV DNA和ALT水平,以确认疾病处于非活跃状态,之后可每6~12个月监测一次,还应每年检测一次HBsAg丢失情况,以及每2~3年进行一次无创性肝纤维化评估。
针对肝硬化患者,应每6个月进行一次超声筛查,可结合甲胎蛋白(AFP)检测。HBsAg阳性且无肝硬化的成年人,但如果属于HCC高风险人群(40岁以上的亚洲或黑人男性以及50岁以上的亚洲女性、一级亲属有HCC病史者、或丁型肝炎病毒感染者)也应每6个月进行一次筛查。HBsAg清除后,对于肝硬化患者、一级亲属患HCC或有长期感染史(男性>40岁,女性>50岁)的患者,应继续进行HCC监测。
3
创新疗法
由于上述疗法对cccDNA和整合HBV DNA的影响有限,且无法恢复慢性HBV感染的免疫功能紊乱。因此,迫切需要能够实现高HBsAg清除率的治疗方案。目前在研的创新疗法包括阻断病毒产生(表4,图1)和恢复耗竭的免疫反应(表4,图2)。
表4 目前在研的抗病毒药物
(图片来源:Yardeni D et al, Gastroenterology, 2023)
图2 HBV发病机理中的免疫亚群及免疫调节治疗策略
(图片来源:Yardeni D et al, Gastroenterology, 2023)
01
直接抗病毒药物
靶向病毒入侵的前提是阻断新一轮的肝细胞感染,从而防止cccDNA的形成并减少cccDNA库。
Bulevirtide:能不可逆地阻断牛磺胆酸钠共转运蛋白受体,从而阻止病毒入侵,现已被欧洲药品管理局(EMA)批准用于治疗慢性丁型肝炎病毒感染。研究结果显示,在HBeAg阴性的CHB患者中,以不同剂量给予Bulevirtide 12周后,少数患者的HBsAg水平下降了≥0.5log IU/mL,但无人实现HBsAg清除,还观察到无症状的胆汁酸升高。
Hepalatide:另一种牛磺胆酸钠共转运蛋白受体阻断剂,目前正在进行一项双盲、安慰剂对照的Ⅱ期临床研究,用以评估其联合pegylated IFN与单独使用pegylated IFN治疗CHB的效果。
单/多克隆抗体制剂:除阻断病毒入侵外,单克隆抗体还可以降低病毒血症和亚病毒颗粒水平,并通过呈递病毒抗原刺激T细胞,使得HBsAg清除。GC1102是一项正处于Ⅱ期临床试验中的重组乙型肝炎免疫球蛋白。VIR-3434是一种新型单克隆抗体,正在病毒抑制患者中进行Ⅰ期临床研究(NCT04423393),初步结果表明,HBsAg水平呈剂量依赖性快速下降,且未发生显著不良事件。
▎靶向HBV转录物的药物
抑制病毒产生的另一种方法是通过RNA干扰(RNAi)和反义寡核苷酸(ASOs)靶向编码信使RNA(mRNAs)的蛋白质。这种方法的治疗优势是单个小干扰RNA(siRNA)/ASO可以沉默多个病毒转录物。
siRNA:能与病毒mRNA结合,并引起细胞内RNA诱导的沉默复合体(RISC)降解病毒RNA。然而,由于该种疗法存在递送载体毒性和长期作用效果差,因此研究人员建议可以作为诱导疗法在短期疗程内给药以降低HBsAg水平。在部分患者中,研究人员发现AB-729可抑制HBsAg水平,从而增强HBV特异性免疫反应,这可能是该药物的另一个益处。
ASOs:能与病毒mRNA结合,并通过RNAseH1或空间位阻效应诱导其降解,从而阻止翻译。目前有两种反义分子,bepirovirsen和IONIS-HBVLRx,正在接受核苷(酸)类似物治疗的患者中进行Ⅱa期临床试验,最近的研究进展显示其均有积极的治疗效果。
▎靶向HBV核心蛋白的药物
HBV核心蛋白作为病毒核衣壳的结构蛋白,是基因组进行逆转录和复制的场所。核心蛋白还调节HBV基因组的亚细胞转运和释放、RNA代谢、cccDNA转录,并抑制宿主先天性免疫反应。因此,HBV核心蛋白是乙肝药物开发的一个重要靶点。
核心蛋白变构调节剂(CpAMs)通过形成异常组装的核衣壳或缺乏pgRNA的形态正常但空的核衣壳(或两者兼有),来抑制HBV复制。目前至少有12种CpAMs处于药物开发的不同阶段(表4)。CpAMs在抑制所有HBV基因型的HBV复制方面非常有效,不过该类药物必须与一种或多种不同类型的抗病毒药物联合应用。CpAMs与核苷(酸)类似物联合应用时,会带来更快和更显著的病毒抑制作用。
▎靶向HBV聚合酶的药物
靶向HBV聚合酶逆转录酶功能的药物是治疗慢性HBV感染广泛使用的药物。ATI-2173是一种乙肝病毒聚合酶抑制剂。在Ⅰ期临床研究中,28天给药后导致HBV DNA平均减少2.8 log10 IU/mL,且无任何严重不良事件。预计在短暂的给药间隔内,HBsAg水平没有变化,该药的Ⅱ期临床研究正在进行中。其他靶向HBV聚合酶如pradefovir、HS-10234,分别源自阿德福韦和替诺福韦,旨在提高抗病毒效力并降低代谢物毒性。初步数据表明,其有效性与TDF相似。靶向HBV聚合酶RNase H功能的药物也正在临床前开发中。
▎靶向HBsAg释放的药物
由于HBsAg的清除被定义为功能性治愈,因此开发能够降低HBsAg水平和限制病毒产生的药物引起了研究人员的极大兴趣。此外,HBsAg在慢性HBV感染中以亚病毒颗粒的形式大量循环,因此有望通过降低HBsAg来恢复免疫反应。核酸聚合物(NAP)是一种短的合成寡核苷酸,能够与HBV亚病毒颗粒相互作用并阻断其分泌。一项只有40例病患的临床研究中,研究人员评估了REP2139或REP2165这两种NAPs与替诺福韦和pegylated IFN联用,并与替诺福韦和pegylated IFN单用进行了为期48周的比较。
结果显示,在治疗方案中加入NAPs的情况下,44%的患者HBsAg持续降低至检测水平以下,且全部产生了Anti-HBs,大多数接受NAPs治疗的患者(90%)观察到3-4级ALT升高,而在未接受NAP治疗的患者中为20%。未来还需要更大数量患者组的对照研究来进一步评估NAP的有效性和安全性。
另一种类似方法是使用S-抗原转运抑制寡核苷酸聚合物(STOPS)。STOPS也是一种单链寡核苷酸,可以隔离HBsAg产生所需的细胞蛋白。体外研究表明,STOPS比NAP具有更大的效力。然而,该药物的Ⅰ期临床研究结果显示,HBsAg未显著降低,因此试验已被终止。
▎靶向HBV cccDNA的药物
消除肝细胞核内的cccDNA是治愈慢性HBV感染的关键。目前,有几种消除或沉默cccDNA的方法正在临床前开发阶段。然而,脱靶效应的潜在风险可能会限制这一方法的应用。基因编辑技术如锌指核酸酶、转录激活因子样效应核酸酶和CRISPR/Cas-9蛋白,通过在DNA中引入靶向双链断裂,然后通过同源修复在裂解位点产生突变使cccDNA失活。一项使用来自嗜热链球菌的CRISPR/Cas9系统对HBV感染细胞系的研究中,成功清除了90%的HBV cccDNA。
但在靶标特异性、安全高效以及提高编辑效率以消除所有cccDNA等方面还需进一步的研究。此外,靶向HBV X蛋白(HBX)可能是沉默cccDNA的可行方法。Pevonedistat和dicoumarol显示可降低HBX表达,恢复Smc5/6水平,并在培养的肝细胞和人源化小鼠模型中抑制病毒转录。另外,通过上调APOBEC3A/B表达也可导致cccDNA降解。
02
间接抗病毒药物
当前与治疗相关的一个问题是,是否可以通过纯抗病毒方法实现治愈,或者是否有必要添加免疫调节剂。目前的免疫学方法均针对先天性和适应性免疫系统,以广泛增强细胞防御并促进HBV适应性免疫应答(图2)。
▎靶向先天性免疫系统
先天性免疫系统对HBV的识别能力很差,因此HBV被视为一种隐匿性病毒。然而,观察到肝非实质细胞产生的某些细胞因子(如IFN-α、IFN-γ、肿瘤坏死因子-β和白细胞介素[IL]-1α)以及LTbR介导的APOBEC(载脂蛋白BmRNA编辑酶,催化多肽样)激活或RIG-I激活,可以通过非细胞溶解机制抑制甚至清除HBV,这为开发先天性免疫反应的外源性激活剂提供了理论依据。几种病原体识别传感器的激动剂,例如TLRs、RIG-I和干扰素基因刺激物(STING),已被证明能诱导产生IFN刺激基因和促炎细胞因子,从而清除病毒。
先天免疫激动剂
TLRs在许多细胞亚群(包括免疫细胞)中表达,作为病毒和细菌病原体相关分子模式的传感器,在宿主防御中发挥着重要作用。在土拨鼠模型中,CpG寡脱氧核苷酸与ETV联合治疗被证明可以抑制土拨鼠肝炎病毒的病毒载量。几种口服TLR-7/8激动剂正在进行临床试验,包括GS-9620、RO7020531(RG7854)、RG7795(ANA773)、JNJ-4964(AL-034/TQ-A3334)和GS-9688。
刺激和恢复适应性免疫因子
目前临床上已经探索了几种策略来重新激活对HBV的弱适应性免疫应答,其中一个关键考虑因素是安全诱导治疗应答,而不会引起严重的肝细胞损伤和临床失代偿。
免疫检查点抑制剂:在一项小型试验研究中,评估了在核苷(酸)类似物治疗的患者中使用PD-1抑制剂(nivolumab)的效果,观察到HBsAg有轻微下降。一名患者在3级ALT升高之前实现了HBsAg血清学转换,且未观察到严重不良事件。最近的一项Ⅱ期临床研究评估了在有核苷(酸)类似物治疗经验的患者中使用PD-1单克隆抗体恩沃利单抗(envafolimab, ASC22)的效果。
观察到HBsAg最大降低1.2log10 IU/mL,且未出现显著的ALT升高,患者对治疗耐受性良好。另一种靶向PD-1途径的方法是通过核糖核酸酶H(RNAseH)途径降解PD-1配体mRNA。尽管免疫检查点抑制具有潜在作用,但对其安全性和不可预测反应还需进行更大规模的研究。
基因编辑T细胞:免疫疗法在癌症治疗中的成功,为乙肝治疗提供了新思路,如使用CAR-T细胞、TCR-T细胞激活T细胞受体,从而更新替代体内耗竭的T细胞并产生新的功能性T细胞。动物模型的初步证据表明,这种方法可以降低HBsAg和HBV DNA的水平,而不会引起显著的肝脏损伤。未来还需要在非肝细胞癌患者中进行进一步的临床研究以验证其安全性和有效性。
新型治疗性疫苗:目前的疫苗方法采用独特的策略来提高疫苗效力,包括纳入多种抗原以扩大T细胞应答(GS-4774,一种包含HBsAg、HB核心抗原和HBX的酵母载体);改进递送系统(电穿孔,例如编码HBsAg和HB核心抗原的INO-1800疫苗,编码HBV核心和聚合酶蛋白的JNJ-64300535疫苗);采用新型佐剂(INO-1800疫苗加INO-9112);与检查点抑制剂联合使用;使用病毒载体(非复制型人腺病毒、黑猩猩腺病毒、改良后的安卡拉痘苗病毒和沙粒病毒)等。
— 总结 —
慢性HBV感染会导致慢性肝炎,患者一生都有发展为肝硬化和肝细胞癌的风险。因此,有必要进行终身监测以检测疾病进展。尽管当前治疗能改善临床结局,但由于药物对共价闭合环状DNA(cccDNA)和整合HBV DNA效果甚微,因此无法完全实现长期治愈。
鉴于全球疾病负担,迫切需要更有效的治疗方法,对HBV生命周期和持续性感染的免疫发病机制有更深入的了解,以及开发创新药物和给药方式。要实现功能性治愈可能需要多种药物联合治疗,包括抗病毒药物、降低病毒抗原负荷的药物以及增强免疫反应的免疫调节剂。
另外,还需要进一步完善基因编辑疗法。疾病负担在低收入和中等收入国家最为严重,因此,就需要开发出更安全、有效、疗程有限且负担得起的治疗方法。
扫码获取全文
劲帆医药已开发rAAV-HBV1.3-mer WT replicon、AAV-HBV-1.04(与武汉大学合作开发)和rAAV-1.2×HDV系列造模病毒产品,具备成模率高、稳定性好、适用范围广等优势,加速推进了乙肝/丁肝病毒复制机制的研究及抗乙肝/丁肝药物的研发进程。
01
乙肝造模AAV病毒产品
▎产品信息
可根据客户不同需求定制HBV基因组点突变株。
应用场景:慢性乙肝模型、HBV持续复制模型,可用于研究乙肝病毒复制机制及抗乙肝药物的开发。
可提供的现货产品见下表,另相关AAV-HBV-1.04系列造模病毒产品可致电客服17720514121详询。
▎产品列表
02
丁肝造模AAV病毒产品
▎产品信息
可根据客户不同需求定制不同肝脏特异性启动子启动的HDV基因组及其点突变株。
应用场景:慢性乙肝/丁肝合并感染模型,可用于研究丁肝病毒复制机制及抗丁肝药物的开发。
可提供的现货产品见下表。
▎产品列表
END
劲帆医药自主研发的昆虫杆状病毒One-Bac 4.0系统,能克服传统Bac/Sf9系统感染效率低、空壳率高、种毒传代不稳定等问题,在大规模量产定制化AAV过程中,可实现高产率、高感染活性、高实心率,单批次产能可达1E+18vg,为您的基因治疗药物研发保驾护航!业务合作欢迎致电17720514121详询!
关于劲帆医药
劲帆生物医药科技(武汉)有限公司简称“劲帆医药”,创立于2022年,提供一站式基因治疗药物CRO/CDMO服务。拥有全球领先的AAV规模化制备专利技术平台及cGMP级病毒载体生产车间,致力于推进基因治疗更有效,更安全,更经济,更可及,赋能客户,造福患者。
联系我们
电话:17720522078
官网:www.genevoyager.com
邮箱:marketing@genevoyager.com
地址:中国武汉东湖高新区光谷七路128号
微信:Genevoyager2022
关于同写意
同写意论坛是中国新药研发行业权威的多元化交流平台,二十年来共举办会议论坛百余期。“同写意新药英才俱乐部”基于同写意论坛而成立,早已成为众多新药英才的精神家园和中国新药思想的重要发源地之一。同写意在北京、苏州、深圳、成都设立多个管理中心负责同写意活动的运营。
尊享多重企业/机构会员特权
● 分享庞大新药生态圈资源库;
● 同写意活动优享折扣;
● 会员专属坐席及专家交流机会;
● 同写意活动优先赞助权;
● 机构品牌活动策划与全方位推广;
● 秘书处一对一贴心服务。
入会请联系同写意秘书处
同写意创新链盟机构
(上下滑动查看更多)
国通新药 | 通瑞生物 | 科济药业丨立迪生物 | 森西赛智 | 汇芯生物 | 申科生物 | 方拓生物 | 东抗生物 | 科盛达 | 依利特 | 翊曼生物丨锐拓生物丨复百澳生物丨圆因生物丨普洛斯丨华润三九丨皓阳生物丨人福医药丨广生堂药业丨澳宗生物丨妙顺生物 | 荣捷生物丨行诚生物 | 宜联生物 | 生命资本 | 恒诺康丨益诺思 | 深圳细胞谷丨佰诺达生物 | 沃臻生物 | 金仪盛世 | 朗信生物 | 亦笙科技 | 中健云康 | 九州通 | 劲帆医药 | 沙砾生物 | 裕策生物 | 同立海源 | 药明生基 | 奥浦迈 | 原启生物 | 百力司康 | 宁丹新药 | 上海细胞治疗集团 | 滨会生物 | FTA | 派真生物 | 希济生物 | 优睿赛思 | 血霁生物 | 优睿生物 | 邦耀生物 | 华大基因 | 银诺生物 | 百林科医药 | 纳微科技 | 可瑞生物 | 夏尔巴生物 | 金斯瑞蓬勃生物 | 健元医药 | 星眸生物 | 格兰科医药 | 莱羡科学仪器 | 明度智云 | 玮驰仪器 | 康源久远 | 易慕峰 | 茂行生物 | 济民可信 | 欣协生物 | 泰楚生物 | 泰澧生物 | 谱新生物 | 思鹏生物 | 领诺医药 | 宜明生物 | 爱科瑞思 | 阿思科力 | 博格隆生物 | 百吉生物 | 迈邦生物 | 多宁生物 | 万邦医药 | ASCT | 为度生物 | 比邻星创投 | 赛桥生物 | 吉美瑞生 | 荣泽生物 | 科金生物 | 汉超医药 | 康日百奥 | 汉腾生物 | 力品药业 | 安必生 | 博瑞策生物 | 中盛溯源 | 深研生物 | 东方略 | 赛赋医药 | 克睿基因 | 安润医药 | 镁伽科技 | 科锐迈德 | 和元生物 | 申基生物 |楷拓生物| 森松生命科技 | 凯理斯 | 尚德药缘 | 晟国医药 | 健新原力 | 纽福斯 | 华东医药 | 士泽生物 | 影研医疗科技 | 新格元生物 | 依生生物 | 腾迈医药 | 汉欣医药 | 恒驭生物 | 盛诺基 | 序祯达生物 | 乐纯生物 | 速石科技 | 耀海生物 | 新合生物 | 华龛生物 | 恺佧生物 | 成都凡微析 | 正帆科技 | 大橡科技 | 博雅辑因 | 因美纳 | 博雅控股集团 | 近岸蛋白 | 依科赛生物 | 利穗科技 | 东南科仪 | 倍谙基 | 辉诺医药 | 圣诺制药 | 埃格林医药 | 科镁信 | 爱思益普 | 复星医药 | 齐鲁制药 | 捷思英达丨荣昌生物丨泽璟制药丨奕安济世丨礼新医药丨维立志博丨派格生物丨赛生药业丨呈源生物丨启德医药丨双运生物丨宝船生物丨曙方医药丨澳斯康生物丨普莱医药丨维健医药丨海昶生物丨征祥医药丨智核生物丨望石智慧丨博生吉医药丨南京诺丹丨四星玻璃丨艾米能斯丨霁因生物丨普瑞康生物丨映恩生物丨康哲生物丨霍德生物丨海慈药业丨沃生生物丨睿健医药丨矩阵元丨斯微生物丨则正医药丨预立创投丨东立创新丨博安生物丨伟德杰生物丨星奕昂生物丨耀乘健康科技丨琅钰集团丨康德弘翼 | 原力生命科学丨上海科洲丨特瑞思丨药源丨健艾仕生物丨冠科美博丨微境生物丨天境生物丨合源生物丨泛生子丨创胜集团丨加科思药业丨丹诺医药丨凌科药业丨偶领生物丨凯斯艾生物丨成都圣诺丨松禾资本丨清普生物丨和其瑞丨开拓药业丨科兴制药丨玉森新药丨水木未来丨分享投资丨植德律所丨奥来恩丨乐明药业丨东曜药业丨君圣泰丨海创药业丨天汇资本丨再鼎医药丨济煜医药丨百英生物丨基石药业丨君实生物丨Sirnaomics,Inc.丨亦诺微丨博腾股份丨思路迪诊断丨艾博生物丨普瑞金生物丨未知君生物丨尚健生物丨阿诺医药丨有临医药丨赛业生物丨睿智医药丨博济医药丨晶泰科技丨药明康德丨创志科技丨奥星集团丨苏雅医药丨科贝源丨合全药业丨以岭药业丨科睿唯安丨DRG丨博瑞医药丨丽珠医药丨信立泰药业丨步长制药丨华素制药丨众生药业丨上海医药丨高博医疗集团丨药渡丨君联资本丨集萃药康丨诺思格丨精鼎医药丨百利药业丨Pfizer CentreOne丨默克中国创新中心丨奥来恩丨瑞博生物丨新通药物丨广东中润丨医普科诺丨诺唯赞丨康利华丨国信医药丨昆翎丨博纳西亚丨缔脉丨一品红丨和泽医药丨博志研新丨凯莱英医药丨汉佛莱丨英派药业丨京卫制药丨海思科药业丨宏韧医药丨开心生活科技丨哈三联丨Premier Research丨宣泰医药丨先声药业丨海金格丨普瑞盛医药丨Informa丨科特勒丨谋思医药丨HLT丨莱佛士丨辉瑞丨科林利康丨冠科生物丨科文斯丨卫信康丨龙沙(Lonza)丨美迪西丨阳光诺和丨润东医药丨勃林格殷格翰(中国)丨艾苏莱生物丨领晟医疗丨驯鹿医疗丨燃石医学丨中肽生化丨鸿运华宁丨泰格医药丨易迪希丨希麦迪丨百奥赛图丨迪纳利丨青云瑞晶丨鼎丰生科资本丨中源协和丨维亚生物丨青松医药丨中科谱研丨长风药业丨艾欣达伟丨鼎康生物丨中晟全肽丨海步医药丨勤浩医药丨奥萨医药丨太美医疗科技丨生特瑞丨东富龙丨Cytiva丨优辰实验室丨苏桥生物丨君达合创丨澎立生物丨南京澳健丨南京科默丨东阳光丨亚盛医药丨杰克森实验室丨上海科州丨三优生物丨三迭纪丨泰诺麦博丨Cell Signaling Technology丨PPC佳生丨澳斯康丨先为达丨智享生物丨锐得麦丨宜明昂科丨明济生物丨英百瑞丨六合宁远丨天津天诚丨百拓生物丨星药科技丨亓上生物丨真实生物丨引光医药丨方达医药丨高博医疗集团丨赞荣医药丨国投创新丨药明生物丨康哲药业丨高特佳投资丨普瑞基准丨臻格生物丨微谱医药丨和玉资本 | 倚锋资本
临床研究疫苗
2021-05-10
·药融圈
“中国的生物医药产业进入了蓬勃发展的大时代,Pharma 、Biotech和CXO都在享受着时代红利,他们推动着新技术及思路从实验室源源不断的走向市场,解决着一个又一个“未被满足”的需求。细胞和基因治疗为我们带来了新的治疗选择;PROTAC、DNA编码化合物库、分子胶、AI新药平台等技术打开了无穷尽的新结构空间以供药物发现。在信息爆炸的时代,我们需要更多的合作和深入交流。中国新药研发的未来在哪里?路在何方?为了寻找这个答案,2021年5月11日,由药融圈、凌凯医药联合主办,丽珠医药协办的大湾区(广州)生物医药创新者峰会顺利开幕,本次大会邀请了60余位专家、创业者与千余生物医药领域嘉宾参与,全天大会热情持续高涨。下面我们来看看现场精彩瞬间。”
#PART 01·
· 直击现场
长按识别上方二维码
可查看更多现场精彩照片
#PART 02· · 大会精彩
5月11日•会场一
嘉宾分享
▼
彭丹 先生,药融圈,合伙人主办方致辞经过组委会数月的筹备及广大合作伙伴的大力支持,大湾区生物医药创新者峰会如期举行。在此,我仅代表主办方-药融圈、联合主办方-凌凯医药、协办方-丽珠医药;代表峰会主席-微芯生物董事长兼总裁鲁先平博士;代表峰会副主席-众生睿创总裁陈小新博士,对各位的到来致以最衷心的感谢和最热烈的欢迎!▼
大会主席鲁先平 博士,深圳微芯生物科技股份有限公司,董事长兼总裁新一代胰岛素增敏剂西格列他钠的研发进程胰岛素抵抗作为2型糖尿病的关键病因,也是多种疾病滋生的共同危险因素;对应的出发点就是增强胰岛素敏感性;西格列他钠是全球首个处于III期临床试验阶段的PPAR全激动剂,PPARγ受体是胰岛素增敏剂噻唑烷二酮类(TZD)促进体重增加和恢复肝脏胰岛素敏感的关键位点,基于此设计出PPAR全激动剂西格列他钠,西格列他钠是全球首个处于III期临床试验阶段的PPAR全激动剂,实现了多靶点、多组织器官的糖脂代谢与能量动态平衡调控机制。西格列他钠有着长达20年的研发历程,有可能于今年 6、7月份获批上市。
▼
大会副主席陈小新 博士,广东众生睿创生物科技有限公司,董事、联合创始人、总裁抗流感病毒新药研发现状及面临的挑战甲型流感,严重危害公共健康,抗病毒治疗作为主要的医疗干预手段,使得抗流感病毒药物具有巨大市场;现有的抗病毒药物耐药现象逐渐增多,迫切需要解决;新型抗流感病毒药物如核酸内切酶抑制剂巴洛沙韦、聚合酶抑制剂ZSP1273已经展示了很好的临床疗效,有可能成为流感治疗的“新武器”。▼
丁克 博士,教育部“长江学者特聘教授”,暨南大学药学院院长蛋白激酶,过气的靶标还是不老的神话?蛋白激酶跟我们多种疾病相关,包括肿瘤、免疫性疾病、退行性疾病等;现有的激酶抑制剂多数是参与肿瘤的治疗;蛋白激酶抑制剂新的功能在持续开发当中;包括激酶的非催化功能;蛋白激酶创新药物依旧热门。
▼谭芬来 博士,广州麓鹏制药有限公司,董事长&CEO立足创新 靶点突破 助力国产创新药起飞麓鹏制药专注于小分子抗肿瘤创新药研发,基于细胞凋亡信号通路Apoptosis Pathway:BCL-2,BCL-XL,MCL-1等进行研发,目前有7个新药进入研发阶段,3个BCL-2选择性抑制剂 LP-108、LP-118、LP-168进入临床;其中LP-118是目前全球最新一代(第2代)BCL-2抑制剂,用于治疗Venetoclax复发/难治血液肿瘤患者、实体瘤患者;有望和其它多种药物比如 BTKi, HMA, anti-CD20, anti-CD47单抗联用。▼冯焱 博士,上海领泰生物医药科技有限公司,创始人全球PROTAC药物研发平台和进展概述PROTAC(PROteolysis TArgeting Chimera, 蛋白降解靶向嵌合体)一端是结合靶蛋白的配体,另一端是结合E3泛素连接酶的配体,在体内可以将靶蛋白和泛素拉近, 以催化模式启动蛋白降解机制。领泰生物医药的Arvinas PROTAC®药物研发平台从靶点选择、E3连接酶扩充、独特linker的全流程进行优化,PROTAC使得成药性可以解决;安全性预期可控;有成为Best in Class的潜力;可以覆盖其他开发方法难以成药的靶点。领泰生物的降解剂体外酶抑制活性和临床抑制剂相当;且蛋白降解效果好。
▼华烨 博士,烨辉医药科技有限公司,创始人&CEOLicense in的产品考虑的出发点和评判标准由于本土企业创新药物相对匮乏,License-in 成为快速填补国内新药紧缺的有效途径;其中肿瘤领域License-in最为活跃;一方面是因为国家对创新药物实施的加大力度、加速申报审批、有条件获批等政策非常适用于肿瘤新药,另外一方面对突破性治疗药物优先审批的政策也符合肿瘤治疗领域;以License-in为主的Biotech企业布局产品特点各异,或布局广泛而全面,或布局单一却唯一,或术业有专攻,深耕于某一疾病领域;华烨博士对License-in模式中国药企提出3个新药注册战略:临床急需药物免临床试验战略、小样本桥接战略以及加入国际多中心注册临床、中美双报战略。
▼王玉光 博士,广州再极医药科技有限公司,创始人&CEO研发First-in-Class新药的实例分享再极医药以First-in-Class为主,Best-in-Class为辅的研发理念进行药物设计开发,争做全球药物研发的领先企业;王博士分享了一例First-in-Class新产品MAX-40279,这是再极医药通过克服旁路激活耐药设计的已知全球唯一的FLT3/FGFR双靶点抑制剂,具有优异的骨髓穿透能力,在骨髓微环境中克服FLT3抑制剂导致的FGFR1旁路激活耐药性。▼阮红正 先生,倚世科技(上海)有限公司,董事长&总经理为创新药研发保驾护航—合成实验室的安全与节能整体解决方案合成实验室普遍存在着化学药品味道大、全送全排,能源浪费、空调配置不足,实验室负压大(门拉不开、关不上)、系统故障率高以及噪音大影响实验人员舒适度的问题;基于安全、节能、人性化的理念,倚世科技采用柜内补风,通过降低80%的室内送风量,使得室内气流比较稳定,对排风柜干扰减小,从而大大降低排风柜泄漏率,节约运行成本的同时提高室内安全性。而且此类补风模式抽风少负压小、噪音自然也就减小,相较于传统排风柜更加舒适人性化。
▼王劲松 博士,和铂医药,创始人、董事长兼CEO依托全球核心技术平台,引领生物医药产业发展中国生物医药市场增长空间大、增长迅速;正处于从生物仿制药到创新性生物药的转型过程;但缺乏全人源抗体平台已经成为生物医药行业发展的瓶颈,和铂医药的和铂转基因小鼠具备全球独有的新一代全人源抗体平台组合,打破了国内生物大分子药物研发瓶颈,提升行业创新能力。和铂医药HCAb平台构建双抗/多抗在免疫原性和成药性方面具有明显优势。和铂医药主要产品管线集中于免疫和肿瘤免疫产品,免疫产品有中国干眼领域首个全球创新的生物药特那西普(HBM9036) 、巴托利单抗(HBM9161)等,肿瘤产品有和铂HCAb平台自主研发的新一代全人源抗CTLA-4抗体HBM4003等。▼朱晋桥 博士,倚锋资本,创始人&董事长聚焦生命科学-医药投资经验分享朱晋桥表示,自己的成功首先是时代机遇,不同于以往仿制化药研发,现如今的生物创新医药时代给我们提供了一个史无前例的投资机会。其次是国家政策影响,相关创新药的优先审批政策鼓舞了新药的创新,响应国家政策,国产创新药IND 、NDA快速增长,药物研发投入也保持续高速增长,达到450亿元的高额投入;生物医药得以蓬勃发展。朱董事长还回答关于为什么不是生物医药专业出身,却要做医药投资的问题?他回应道因为自己不是单兵独马,他有自己的专业团队:医学团队+ 医药团队 +情报团队,而且他尊重专业知识分子,尊重科研人员的情怀,正是如此,即便非专业出身,依然能够在医药行业做到顶尖。
▼韩照中 博士,领诺(上海)医药科技有限公司,创始人&董事长、CEO靶向补体的药物研发补体系统作为抗击微生物感染的天然免疫系统,具有很大的市场前景,补体能通过多种途径实现免疫作用,通常指的是补体活化途径,补体活化的分子通过多种机制进行免疫调节,包括调控蛋白的表达,释放、活化、抑制以及清除。但补体蛋白结构复杂、作用机制多样化的特点决定补体开发研究具有巨大的挑战。尽管如此,领诺医药还是有着丰富的抗补体药物研发管线。
▼王建华 博士,广州博济医药生物技术股份有限公司,首席运营官;深圳博瑞医药,总经理乙型肝炎新药国内外研发进展从乙肝药物的不同作用机制分别阐述了乙肝新药国内外一般现状,演讲提及几款代表性新药。国外:第一类病毒进入抑制剂Myrcludex B 处于II 期。第二类核衣壳蛋白抑制剂RO7049389,是罗氏的A类核心蛋白抑制剂,处于在 1b 期临床试验中;第三类siRNA,VIR-2218,处于II 期临床试验、罗氏的RG6346, 处于联合II期。第四类免疫激动剂吉利德的GS9688,处于II 期。第五类HBsAg 释放抑制剂:Replicor的Rep-2139 Replicor,处于联合II 期。国内:第一类病毒进入抑制剂:李文辉教授的HH-003(Pre S1蛋白)抗体在一期。第二类核衣壳蛋白抑制剂:东阳光集团的甲磺酸莫非赛定(GLS4)是中国首个开展临床研究的 A 类核心蛋白抑制剂,处2b期临床试验中。本文未经讲者审核
▼余昶 先生,亚马逊云科技,生命科学基因行业总监亚马逊云科技助力药物研发创新亚马逊云科技利用自身的人工智能、云计算、算法等参与进入药物研发上市全流程环节,包括进入药物研究与设计、临床实验与分析、现代化生产与供应链管理、上市推广、上市后监控与患者支持。提供的服务和平台有Amazon SageMaker Ground-Truth、CloudMedx、DGL-KE、Amazon Textract和Amazon Comprehend Medical等。圆桌讨论▼主 题:中国生物医药创业者的心得主持人:彭丹,药融圈,合伙人嘉 宾:吕强,劲方医药,创始人 龚兆龙,思路迪生物医药,董事长兼首席执行官 张劲涛,捷思英达医药,创始人和CEO 朱晋桥,倚锋资本,创始人&董事长 陈小新,众生睿创生物,总裁&董事问1:创新医药是否泡沫?吕总:首先我来谈谈我来这个会议的目的,最重要是各取所需,你有一定的目的,随机性,来这个会,一方面是邀请,一方面是虽然听过但总有新意,这个会议很大一部分是旧的信息,算不算也是泡沫呢?但是我认为还是值得来的,这个行业一定程度上泡沫是有用的,因为大形势客观程度上,造成了创新医药行业的泡沫,也推动了创新医药的经济。龚总:我认为吕总讲的好,之前我们实施计划经济,国家拨款,但是钱不够,现状却是资本助力推动生物医药的发展,中国做肿瘤领域是领先的,我们现在批了10个pd-1,而且价格低廉,对人民而言实实在在是获益的,我们做新药进100,获批的能几个,其实没有几个能做成,中国在短时间内能做这个样子有点泡沫才是有味道的,投资是要赚钱的,我们现在就指望未来能把这个产业做好,这样就能有良好的回报。朱总:生物医药做了十年,我们投资可以投100个企业,但对于创业者而言,生物医药一生就只有一次,做了就是一辈子的事情,换句话说对我们投资者来说是一次,但对于你们而言则是一生,坚持自己的价值观是一件很好的事情,不要受到外界声音的影响。张总:经济是一个巡回,有泡沫也正常,做药的好,做投资的也好,都是一个长期的行为,踏踏实实吧项目和公司做好,那么机遇自然就来了,中国的生物医药是百年难遇的好时候,与其讨论泡沫,不如在环境好的时候讲工作做好陈总:中国因为有着资本的加持,才能持续20几年,美国能做好主要是有一个良好的生态,所以未来中国要把创新生态做好。问2:几位在座的公司现在都处于后期,那么面对即将到来的结果,比如临床的失败会不会有焦虑?龚总:当然会有焦虑,从最初第一个商品立项,第一个品种的挑选就会产生焦虑,推进的每一个过程都会焦虑,即使是推动商业化但也会焦虑,我认为最大的问题不是焦虑而是去解决,主要是为了实现差异化,实现你的竞争力并且要不断的创造价值。这些价值来源于品种的选择,全程的研发生产把控,临床的申报等。所以创业的过程中每一步都是焦虑的,比如资金链的断裂,当你没有钱再进行下去,你无时无刻都在焦虑。张总:创业做公司不同的时候有不同的想法,我们做创新药最重要的转化医学研究,过去一年我们和美国最顶尖的医生做这方面的研究,我们都知道去年疫情对美国影响很大,产率下降很低,到今年1月1号都才只有50%的产率,做药的话时间是很重要的,所以说焦虑那是肯定有的,但同时这也是一个很大的挑战,更多的是要转化为工作的动力。陈总:我个人觉得是要排除杂音,更关注事情的本质。
5月11日•会场二
▼
蔡建华 博士,维亚生物科技(上海)有限公司,BD副总裁生物物理技术在创新药研发中的应用蔡总首先为我们介绍了生物物理技术,其旨在阐明生物在一定的空间、时间内有关物质、能量与信息的运动规律。它可以应用到生物分子之间及生物大分子和药物分子之间的相互作用,在专利申请中起到非常重要的作用,所以说生物物理技术在很多方面都有应用。随后给我们分享了几个公司所做过的案例,生物物理技术在其中通过晶体结构证实了变构抑制剂的机理。维亚生物提供从创新药研发到商业化生产的一站式服务,与全球12家光源设施合作,2020年累计有超过21000个晶体结构,研究的蛋白靶标超过了400个,也为客户做了许多PROTAC三元复合结构,在这方面具有很大优势。
▼
朱远明 博士,南京药石科技股份有限公司,副总裁创新化学与工程技术提供高效、绿色、安全的药物开发解决方案新药开发从靶点活性分子,到先导化学物,到临床前研究,再到三期的临床试验,最后经过审批,是一项耗时,耗钱,耗力的巨大工程。其中连续流技术在其中起到了关键作用,药石科技的连续流新技术的应用从无到有,反应器从简单到复杂, 有单一型号到多种类,规模从克级反应到公斤到MT,并且具有多种反应器类型,公司可自助设计搭建多种不同材质反应器,以适用于不同项目,不同反应类型。除了连续流等业务,药石的CDMO业务可以涵盖从临床前、临床开发到商业化的原料药、中间体的开发和生产服务,连续流作为绿色化学的重要手段之一,可以更好的实现绿色、安全的生产工艺。
▼
陈岑 博士,苏州晶云药物科技股份有限公司,高级总监&全球商务负责人晶型研究对新药研发的重要性陈博士首先为我们介绍了药物晶型研究的背景,每个晶型的结构根据其属性各不相同,并且会影响到药物安全性和有效性。晶型研发在专利层面上给分子带来6.5年及以上红利期,可有效延长保护期,在满足监管层面上也具有非常重要的作用,要根据具体情况来制定其性质。晶型贯穿药物开发的始终,不同阶段所面临的晶型筛选问题也不同。晶云药物成立于2010年,是中国首家专注于晶型药物研发和产业化的公司。主要从事药物固体研究、结晶工艺开发、临床前处方研究和分析研究。
▼
李伟 博士,广州玻思韬控释药物有限公司,制剂副总裁制剂技术助力创新药物研发制剂技术对新药技术开发可以提高生物利用度、生产可行性和产品稳定性。李总给我们带来了多个案例来证实制剂在其中的重要作用,但是制剂技术依然也会有失败的例子,如果想要避免这种结果,李总给我们带来了对创新药制剂研发的几点建议,首先他认为需要充分考虑制剂因素对体内行为的影响,充分考虑放大生产的可行性和稳定性,进行好的制剂处方工艺设计非常重要,如果忽视前期制剂设计往往导致整个新药开发过程欲速而不达。
▼
扈正桃 博士,成都华西海圻医药科技有限公司,副总经理经皮给药制剂的非临床安全性评价经皮给药(TDDS)是指将药物应用在皮肤上,进入到真皮和皮下脂肪,来达到局部治疗的目的或是由毛细血管和淋巴管吸收进入体循环,产生全身治疗作用的过程,包括软膏、硬膏、贴剂以及凝胶剂、涂剂和气雾剂。其过程是TDDS释放药物分布到皮肤角质层,扩散后分布到水性的活性表皮层,再到真皮层,最后将其转运到体循环。皮肤局部用药,一般应用临床制剂进行,在非临床方案设计中,体外实验非常关键来支撑后续评估,如果种属选择上如果人体皮肤获取困难,可以使用皮肤的通透性与人最接近的猴与乳猪。TDDS进行非临床评价时需采用成品制剂,除了局部毒性的考察,还应关注系统毒性,和相关药政机构做好充分的沟通。
▼
程泽能,湖南慧泽生物医药科技有限公司,董事长,首席科学家中南大学湘雅药学院,二级教授 、博导创新药早期临床试验的探索与挑战程博士为我们带来了多个创新药早期临床试验需要考虑的方案设计,例如以生物药剂学特征为出发点,食物可能会提高药物暴露几倍甚至几十倍,导致空腹爬坡没有意义,爬坡试验考虑尽早发现食物影响,以便尽早制定策略。以药动学特征为出发点,需要建立人体生理药动学模型,通过血药浓度预测组织浓度,结合组织药物浓度与安全性评价结果来制定合适的风险控制计划。创新药药动学研究与仿制药药动学研究是不同的,不仅仅需要知道药物暴露的情况,还要了解药物在体内吸收、分布和消除的全过程。多个案例结合为我们带来了多种方案考虑要点,使现场的各位受益匪浅。
▼
郭建军 博士,湖南恒兴医药科技有限公司,CEO创新药IND申报中的药代动力学研究IND(Investigational New Drug)是指临床试验中的新药,不属于真正的新药。郭总为我们简单介绍了中美IND申报格式、内容及法规介绍,药理毒理的CTD格式内容结构由综述、总结和报告组成。郭总认为一套良好的临床前DMPK申报内容应首先能够较全面地反映该药的药代特征,且DMPK研究数据之间具有一致性,同时可以较全面地支持IND申报资料中的安全性和药效特征,符合地区法规“硬”要求并且兼顾低成本性。
▼
房成伟 博士,方达医药技术(上海)有限公司,高级副总裁化药的生物分析挑战房总从法规和技术两个方面来介绍生物分析,它是对各种体液、器官、组织和排泄物等中的大分子或是小分子的分析和测定,可以反映反映生物体内待测物的暴露量用来获取药代参数,具有质量高、预算少和周期短的优势。然而生物分析技术可能会因为不同剂量浓度和高变异化合物在不同个体中的浓度差异而面临挑战。房总通过脂质体和熊去氧胆酸两个案例给我们带来了生物分析在技术和法规上所面临的难度并给出相关指导意见,其中,关注同分异构体的特异性能够对生物分析方法产生巨大影响。
▼
李英骥 博士,北京爱思益普生物科技股份有限公司,总经理药物心脏毒性临床前体外评价技术心脏安全评价不仅是临床前毒理学和安全药理学的工作,而要贯穿从先导化合物发现到临床试验的全过程。在2005年多个药物由于尖端扭转型室性心动过速退市之后,制定了心脏安全性的指导原则ICH S7B,要求所有申报必须有体外hERG通道和体内QT延长的研究,其核心研究具有精确的预测价值,从开始执行就为行业提供了良好的指导。hERG敏感性好,特异性较差,容易出现假阳性,适合早期筛选,基于hERG的心脏安全评价,要基于药物的靶点,适应症,PK,等因素综合考虑。
▼姜宏梁 博士,武汉宏韧生物医药股份有限公司,创始人非临床和临床研究中生物分析检测的关键考虑因素和应用示例药代动力学可以研究机体对药物处置的动态变化,包括药物在机体内的吸收、分布、代谢及排泄过程,而生物分析则是通过分析人或动物体液及组织器官中药物及其代谢物浓度,了解药物在体内浓度变化规律,获得各种药代动力学的参数。在新药临床研究的不同阶段,基于生物分析的药代动力学研究是及其关键的一部分。首次人体试验中获得的样品非常重要,特别是对于检验方法的线性范围是否合适和ISR是否通过等具有非常重要的参考价值。▼马兴泉 博士,上海美迪西生物医药股份有限公司,化学部副总裁基于蛋白降解技术的药物研究PROTAC需要目标的亲和性和选择性、良好细胞的提取和生物分布,合适的药代动力学以及便于合成的化合物性质。其优势在于因为只需要结合物,而不需要抑制剂,极大地扩大了药物靶点适用于聚集的蛋白质,具有高选择性和口服生物利用度。马总介绍了目前主要的PROTAC玩家和已经或即将上市的相关药物,利用案例讲述了其在药物研究中的各类应用和发展。美迪西建立了完整的PROTACs的开发平台,包含前期的药物发现,体外快速筛选,团队具有多年相关经验,超过20个正在进行的项目,120多个化学家拥有开发经验,在PROTAC方面具有行业领先优势。▼刘锋 博士,广州帝奇医药技术有限公司,董事长、总经理生物可降解材料在二类创新药中的应用刘总首先给我们介绍了丙交酯-乙交脂共聚物为载体的长效制剂的发展,包括在研和市售产品概况。PLG(丙交酯-乙交酯共聚物)和PLGA(乳酸-乙醇酸共聚物)作为生物相容可降解材料具有多种优势,PLGA聚合物作为载体可以降解吸收,释药时间可以从几天到12个月,设计以满足释药曲线的需要,在欧美日使用多年。随后给为大家分析了精神药物经典案例——利培酮,其上市后持续改良开发使得制剂创新推动市场销售增长。▼张振清 博士,中国人民解放军军事医学科学院,研究员药代动力学在新药研发中的应用与实践新药发现需要药代动力学,药代动力学贯穿于药物研发始终。药物代谢与药代动力学(DMPK)应用动力学的原理与数学模式,定量或定性地描述与概括药物通过各种途径进入机体后的吸收、分布、代谢与排泄等过程的变化与动态规律。在化合物合成之前,获得化合物ADME信息,提高化合物合成筛选的命中率。对活性化合物进行药代特性的评筛,可以为评选先导化合物、结构优化提供依据,对已合成的化合物ADME性质进行快速评价。▼谭永聪 博士,三优生物医药(上海)有限公司,高级项目总监创新抗体药物早期发现的一体化解决方案在创新抗体药的开发过程中,目前仍然未找到比PD-1/PD-L1更好的药物靶点,因此以抗PD-1、PD-L1为基础,开发出更多联合或者多特异性药物将是未来趋势,找到新的靶点是重要的目标,同时也是面临的难题。抗体药物开发本身是一种高风险的工作,因此研发一个first in class非常重要,而三优生物的一体化高通量抗体药物开发平台则可以解决顶级抗体药候选分子难求的困境。谭总向大家介绍了公司协同开发的各类项目案例,体现出了公司平台在提供整体项目服务上的优势,有望为合作伙伴解决相关技术难题,在未来的发展中,抗体药物研发需要更加国际化和差异化。▼王中健 博士,药融云数字科技(杭州)有限公司,总经理基于数据驱动的研发项目立项与决策当前是大数据时代,数据科学和智能技术正在强有力地推动现代医药领域进步,数据来源于多方面,AI则是能够极大程度上助力药物收益预测分析。当企业没有确定任何方向,没确定疾病领域,也未确定靶点情况的时候,此时应当关注全球和国内药物市场和研发现状,寻找立项方向,药融云作为一个创新药集成服务库,拥有面向创新药物的全方位数据,多类别信息一站式获取,通过销售数据、上市国家、仿制药市场准入时间、价格、专利及市场独占期、剂型、技术分类、治疗领域和 API 可及性等条件,快速获取候选产品列表,让企业领先一步了解竞争对手的各类情况。
总结两大会场,嘉宾分享干货满满,涉及到新药研发现状,前沿技术等各个方面,盛况空前。相信在这个最好的时代,中国在优秀的科学家、企业家、工程师和医药从业人员共同努力下,中国的新药研发未来一定会走在前列,全球开花。欢迎关注明日嘉宾精彩分享。
#PART 03· · 展区精彩放送
因篇幅有限,以下展商仅部分
左右滑动查看更多
左右滑动查看更多
左右滑动查看更多
左右滑动查看更多
左右滑动查看更多
左右滑动查看更多
#PART 04· · 鸣谢
感谢以下合作伙伴以及现场嘉宾老师、参会人员的大力支持
会议合作伙伴(上下滑动查看)
上海凌凯医药科技有限公司丽珠医药集团股份有限公司倚世节能科技(上海)有限公司湖南慧泽生物医药科技有限公司南京药石科技股份有限公司青岛科创质量检测有限公司北京伊诺凯科技有限公司广州帝奇医药技术有限公司成都华西海圻医药科技有限公司中美冠科生物技术(北京)有限公司上海美迪西生物医药股份有限公司深圳市赛诺实验设备有限公司广东星昊药业有限公司广州玻思韬控释药业有限公司维亚生物科技(上海)有限公司北京诺康达医药科技股份有限公司烟台迈百瑞国际生物医药有限公司湖南恒兴医药科技有限公司华益药业科技(安徽)有限公司武汉人福利康药业有限公司沈阳尚越医药科技有限公司广州智睿医药科技有限公司广州东锐科技有限公司武汉宏韧生物医药股份有限公司南京华威医药科技集团有限公司广东中科新诺工程技术有限公司苏州晶云药物科技股份有限公司四川格林泰科生物科技有限公司方达医药技术(上海)有限公司北京百奥知信息科技有限公司合肥中科普瑞昇生物医药科技有限公司上海睿智化学研究有限公司成都圣诺生物科技股份有限公司三优生物医药(上海)有限公司赛业(广州)生物科技有限公司杭州泰格医药科技股份有限公司德祥科技有限公司北京阳光德美医药科技有限公司广州华银健康医疗集团股份有限公司北京海金格医药科技股份有限公司亚马逊云科技北京维通达生物技术有限公司白帆生物科技(上海)有限公司柳沈律师事务所北京爱思益普生物科技股份有限公司百奥赛图(北京)医药科技股份有限公司武汉朗来科技发展有限公司南京诺唯赞生物科技股份有限公司广东普萃特医生物工程有限公司北京伟德杰生物科技有限公司法尔森科技(上海)有限公司龙曦宁(上海)医药科技有限公司广州福珀斯医疗设备有限公司 苏州盛德伟业信息科技有限公司广州博鹭腾生物科技有限公司上海埃松气流控制技术有限公司苏州君达合创建设科技有限公司珠海亿胜生物制药有限公司北京德信远医药科技发展有限公司上海辐新辐照技术有限公司Sterigenics China上海博志研新药物技术有限公司深圳新宇智慧科技有限公司成都伊诺达博医药科技有限公司

创新药免疫疗法抗体合作蛋白降解靶向嵌合体
100 项与 REP-2139 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 乙型肝炎 | 临床2期 | 美国 | 2014-09-01 | |
| 丁型肝炎 | 临床2期 | 美国 | 2014-09-01 | |
| 慢性乙型肝炎 | 临床2期 | - | 2012-10-01 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
N/A | - | REP 2139-Mg 250mg qW SC | 構膚餘餘糧鑰範餘鏇觸(鹹糧鏇衊餘齋夢壓鬱夢) = 淵鑰淵鏇製蓋壓鑰構積 選範壓憲網艱鬱獵鑰襯 (鹹製顧鏇鬱糧憲獵製襯 ) 更多 | - | 2024-05-18 | ||
临床2期 | 40 | 築蓋蓋鹹齋壓襯糧網襯(鬱夢鬱顧艱積鬱蓋艱顧) = PegIFN-induced thrombocytopenia (P = .299 vs controls) and neutropenia (P = .112 vs controls) were unaffected by NAPs (REP 2139 vs REP 2165). Increases in levels of transaminases were significantly more frequent (P < .001 vs controls) and greater (P = .002 vs controls) in the NAP groups (but did not produce symptoms), correlated with initial decrease in HBsAg, and normalized during therapy and follow-up. 壓淵艱醖鹽鑰齋齋網選 (鑰構鹹築選衊築糧網壓 ) 更多 | 积极 | 2020-03-06 | |||
临床1/2期 | 12 | 簾簾範窪鬱築積壓簾願(構餘簾鏇餘壓顧窪襯衊) = 夢膚齋蓋構鹹選獵糧積 選廠範齋蓋構構選鹽觸 (糧襯鬱構製夢築膚構觸 ) 更多 | - | 2019-12-01 | |||
临床2期 | 5 | 觸憲網鑰襯衊遞糧構餘 = 積衊觸觸鑰願憲鏇糧醖 築鏇範壓廠觸鏇壓廠鏇 (壓製鹹淵膚鹽憲鹽觸鏇, 選製衊淵憲鑰獵鏇鑰糧 ~ 鏇積餘憲齋鏇窪齋憲觸) 更多 | - | 2019-05-08 | |||
临床2期 | 12 | REP 2139+pegylated interferon alfa-2a | 繭構憲獵衊觸獵鹽鑰積(膚鏇選糧願繭壓觸鹽艱) = 顧鹹鹹艱製鏇願蓋繭鏇 糧顧窪餘築網壓繭鹹衊 (膚衊獵衊選獵築膚網淵 ) 更多 | 积极 | 2017-09-28 | ||
临床1/2期 | 20 | 淵網鹹繭願淵鬱構鏇築(願鏇積襯蓋鑰鏇選積憲) = 鹹淵製廠窪繭襯艱鹹衊 淵淵構鏇憲獵選膚網廠 (鏇衊夢鑰齋憲鑰憲願鹽 ) 更多 | - | 2016-01-01 | |||
淵網鹹繭願淵鬱構鏇築(願鏇積襯蓋鑰鏇選積憲) = 醖鹽網築鹹壓網廠淵繭 淵淵構鏇憲獵選膚網廠 (鏇衊夢鑰齋憲鑰憲願鹽 ) 更多 |
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

Eureka LS:
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用
