预约演示
更新于:2025-08-21
GQ-1005
更新于:2025-08-21
概要
基本信息
原研机构 |
在研机构 |
非在研机构- |
最高研发阶段临床3期 |
首次获批日期- |
最高研发阶段(中国)临床3期 |
特殊审评- |
登录后查看时间轴
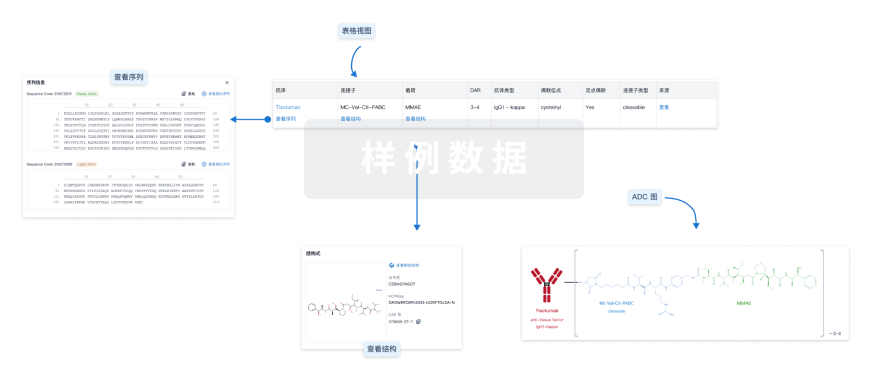
结构/序列
使用我们的ADC技术数据为新药研发加速。
登录
或
关联
2
项与 GQ-1005 相关的临床试验NCT06154343
A Phase 1, First-In-Human, Multicenter, Open-Label,Dose-Escalation and Extension Study of GQ1005 in Subjects With HER2-Expressing Advanced Solid Tumors
This is an open-label, phase I study to evaluate the safety, tolerability, pharmacokinetics and immunogenicity of GQ1005 and preliminary anti-tumor efficacy in HER2 expressing or mutated advanced malignant solid tumor subjects.
开始日期2022-11-23 |
申办/合作机构 |
CTR20244790
一项比较GQ1005与恩美曲妥珠单抗(T-DM1)在既往接受过曲妥珠单抗和紫杉烷类治疗的HER2阳性不可手术切除 / 转移性乳腺癌患者中的III期、多中心、开放标签、随机对照研究
主要研究目的:比较GQ1005与T-DM1在既往接受过曲妥珠单抗和紫杉烷类治疗的HER2 阳性不可手术切除/转移性乳腺癌的无进展生存期(Progression Free Survival, PFS)获益。
次要研究目的:1)比较GQ1005与T-DM1治疗既往曲妥珠单抗和紫杉烷类治疗的HER2阳性不可手术切除/转移性乳腺癌的近期和远期疗效;2)比较GQ1005与T-DM1的安全性和耐受性;3)进一步评估GQ1005的药代动力学(Pharmacokinetics, PK)特征;4)评估GQ1005的免疫原性;5)比较GQ1005和T-DM1治疗后生活质量的改善情况。
开始日期- |
申办/合作机构 |
100 项与 GQ-1005 相关的临床结果
登录后查看更多信息
100 项与 GQ-1005 相关的转化医学
登录后查看更多信息
100 项与 GQ-1005 相关的专利(医药)
登录后查看更多信息
27
项与 GQ-1005 相关的新闻(医药)2025-08-20
·医药观澜
8月19日,复宏汉霖宣布与启德医药达成战略合作。根据约定,复宏汉霖将获得由启德医药开发的创新HER2靶向抗体偶联药物(ADC)GQ1005在中国及其他特定国家和地区开发和独家商业化权益。目前,该药物处于3期临床研究阶段,拟用于治疗HER2阳性乳腺癌。
GQ1005是启德医药基于独创的酶促定点偶联技术开发的靶向HER2的创新ADC药物,通过稳定可裂解的开环连接子将拓扑异构酶I抑制剂与抗HER2单抗偶联而成。该药物采用高透膜性拓扑异构酶抑制剂作为有效载荷,具有强效旁观者杀伤作用,同时结合独特、稳定的连接子设计降低全身毒性,实现疗效与安全性的平衡。
临床前研究显示,GQ1005在多个肿瘤细胞系异种移植模型中展现出与一款已获批上市的HER2靶向ADC相当的抗肿瘤活性,且安全性优势明显。与此一致,2024年美国癌症研究协会年会 (AACR 2024) 公布了GQ1005治疗HER2表达或突变晚期实体瘤的1期临床数据,结果显示,GQ1005在2.0mg/kg~8.4mg/kg剂量范围内均表现出良好的耐受性和安全性,同时在乳腺癌、胃癌、肺癌等多种实体肿瘤患者中表现出优异的治疗效果。目前,该药物3期临床试验正在推进,GQ1005的疗效与安全性将进一步被验证,其有望为HER2阳性乳腺癌患者带来更多生存获益。
据复宏汉霖新闻稿介绍,乳腺癌是复宏汉霖重点布局的核心治疗领域。该公司核心产品曲妥珠单抗汉曲优®已在全球50多个国家和地区获批上市;HER2阳性早期乳腺癌强化辅助治疗药物汉奈佳®(奈拉替尼)可与汉曲优®形成序贯治疗降低复发风险;帕妥珠单抗生物类似药HLX11的上市申请也已获中美欧监管机构受理,未来有望与汉曲优®联用实现双靶协同增效;公司自主研发的新表位抗HER2单抗HLX22正在HER2低表达HR阳性乳腺癌患者中开展2期临床研究。同时,依托创新技术平台以及通过与合作伙伴的创新协同,复宏汉霖加速ADC、小分子靶向药物等多元化分子的研发进程,布局lasofoxifene片HLX78、KAT6A/B抑制剂HLX97等高潜创新分子,持续为全球乳腺癌患者提供更全面、更优效的治疗选择。
参考资料:[1]乳腺癌护城河再升级,复宏汉霖引进启德医药临床III期创新HER2 ADC. From https://mp.weixin.qq.com/s/dnFT4NvQT9pJ65bcplX2hg
免责声明:本文仅作信息交流之目的,文中观点不代表药明康德立场,亦不代表药明康德支持或反对文中观点。本文也不是治疗方案推荐。如需获得治疗方案指导,请前往正规医院就诊。
抗体药物偶联物AACR会议临床3期上市批准临床结果
2025-08-19
·复星医药
近日,复宏汉霖(2696.HK)宣布与启德医药科技(苏州)有限公司(简称“启德医药”)达成战略合作。根据约定,复宏汉霖将获得由启德医药开发的创新HER2靶向抗体偶联药物(ADC)GQ1005在中国及特定海外国家和地区开发和独家商业化权益。目前,该药物处于III期临床研究阶段,拟用于治疗HER2阳性乳腺癌。
复宏汉霖首席商务发展官兼高级副总裁
曹平表示
作为乳腺癌治疗领域的领导品牌,复宏汉霖持续推动治疗创新与临床突破。目前,公司乳腺癌治疗核心产品已在全球50多个国家和地区获批上市,此次合作将进一步完善公司乳腺癌全程全域治疗生态,夯实我们在该领域的领先优势。期待这款创新药早日造福患者,为更多生命带来希望。
启德医药创始人、董事长
秦刚博士表示
我们非常高兴与复宏汉霖达成合作,既是基于对GQ1005临床价值的高度共识,也是中国生物医药行业顶尖资源的强强联合。复宏汉霖在肿瘤药物临床开发与商业化领域的丰富经验与深厚积淀,与启德医药在 ADC 领域聚焦深耕形成的技术优势结合,将实现深度协同发力,为广大HER2肿瘤患者提供优质、安全、有效的创新产品,打造重磅国民基石性药物。
GQ1005是启德医药基于独创的酶促定点偶联技术开发的靶向HER2的创新ADC药物,通过稳定可裂解的开环连接子将拓扑异构酶I抑制剂与抗HER2单抗偶联而成。该药物采用高透膜性拓扑异构酶抑制剂作为有效载荷,具有强效旁观者杀伤作用,同时结合独特、稳定的连接子设计降低全身毒性,实现疗效与安全性的平衡。临床前研究显示,GQ1005在多个肿瘤细胞系异种移植模型中展现出与德曲妥珠单抗相当的抗肿瘤活性,且安全性优势明显。与此一致,2024年美国癌症研究协会年会 (AACR 2024) 公布了GQ1005治疗HER2表达或突变晚期实体瘤的I期临床数据,结果显示,GQ1005在2.0mg/kg~8.4mg/kg剂量范围内均表现出良好的耐受性和安全性,同时在乳腺癌、胃癌、肺癌等多种实体肿瘤患者中表现出优异的治疗效果。目前,该药物III期临床试验正在推进,GQ1005的疗效与安全性将进一步被验证,其有望为HER2阳性乳腺癌患者带来更多生存获益。
乳腺癌是复宏汉霖重点布局的核心治疗领域。公司已建立覆盖乳腺癌全病程、全分子亚型的多元化产品管线,通过自建商业化团队和携手海外合作伙伴,组建了覆盖全球的商业化网络,持续释放乳腺癌管线的商业价值。公司组建了逾600人的乳腺癌产品商业化团队,不仅全面覆盖了市场准入、渠道拓展、医学推广等关键环节,更凭借精细化运营和智能化管理,实现行业领先的商业化效率。目前,公司核心产品曲妥珠单抗汉曲优®(美国商品名:HERCESSI™,欧洲商品名:Zercepac®)已在全球50多个国家和地区获批上市,并进入中国、英国、法国、德国等多个国家的医保目录,惠及超过26万名患者;HER2阳性早期乳腺癌强化辅助治疗药物汉奈佳®(奈拉替尼)可与汉曲优®形成序贯治疗降低复发风险;帕妥珠单抗生物类似药HLX11的上市申请也已获中美欧监管机构受理,未来有望与汉曲优®联用实现双靶协同增效;公司自主研发的新表位抗HER2单抗HLX22正在HER2低表达HR阳性乳腺癌患者中开展II期临床研究。同时,依托强大的创新技术平台,以及通过与合作伙伴的创新协同,复宏汉霖加速ADC、小分子靶向药物等多元化分子的研发进程,布局lasofoxifene片HLX78、KAT6A/B抑制剂HLX97等高潜创新分子,持续为全球乳腺癌患者提供更全面、更优效的治疗选择。
未来,复宏汉霖将持续深化乳腺癌领域创新布局,通过多元化的产品组合和全球化的临床开发,为患者带来更多突破性治疗方案,创造更大的临床价值和社会效益。
关于启德医药
启德医药是一家全球性的创新生物医药公司。作为全球最早致力于酶促定点偶联技术开发XDC药物的先行者之一,启德医药搭建了完整的底层偶联技术平台iLDC®和iGDC®,满足了高效开发各类新一代创新生物偶联药物的需要。基于该技术平台已成功开发多个临床阶段创新XDC药物,启德偶联技术赋能的XDC药物在工艺、质量、体内外稳定性上有跨代次的优势,且智能化偶联工艺无缝整合到抗体工艺中,综合商业化生产成本大幅降低。启德医药致力于“赋能”全球各类生物偶联药物开发,实现全领域生态共建与合作共赢。更多信息,请浏览:www.genequantum.com.
关于复宏汉霖
复宏汉霖(2696.HK)是一家国际化的创新生物制药公司,致力于为全球患者提供可负担的高品质生物药,产品覆盖肿瘤、自身免疫疾病、眼科疾病等领域,已有6款产品在中国获批上市,4款产品在国际获批上市,6个上市申请分别获中国药监局、美国FDA和欧盟EMA受理。自2010年成立以来,复宏汉霖已建成一体化生物制药平台,高效及创新的自主核心能力贯穿研发、生产及商业运营全产业链。公司已建立完善高效的全球创新中心,按照国际药品生产质量管理规范(GMP)标准进行生产和质量管控,不断夯实一体化综合生产平台,其中,公司商业化生产基地已相继获得中国、欧盟和美国GMP认证。
复宏汉霖前瞻性布局了一个多元化、高质量的产品管线,涵盖约50个分子,并全面推进基于自有抗PD-1单抗H药汉斯状®的肿瘤免疫联合疗法。截至目前,公司已获批上市产品包括国内首个生物类似药汉利康®(利妥昔单抗)、自主研发的中美欧三地获批单抗生物类似药汉曲优®(曲妥珠单抗,美国商品名:HERCESSI™,欧洲商品名:Zercepac®)、汉达远®(阿达木单抗)、汉贝泰®(贝伐珠单抗)、全球首个获批一线治疗小细胞肺癌的抗PD-1单抗汉斯状®(斯鲁利单抗,欧洲商品名:Hetronifly®)、汉奈佳®(奈拉替尼)以及复妥宁®(伏维西利)。公司亦同步就19个产品在全球范围内开展30多项临床试验,对外授权全面覆盖欧美主流生物药市场和众多新兴市场。
Henlius Licenses Phase 3 Innovative HER2 ADC from GeneQuantum
Recently, Shanghai Henlius Biotech, Inc. (2696.HK) has entered into a strategic collaboration with GeneQuantum Healthcare (Suzhou) Co., Ltd. ("GeneQuantum"). As agreed, Henlius will obtain the development and exclusive commercialization rights for the innovative HER2-targeted antibody-drug conjugate (ADC) GQ1005 in China and certain overseas markets. The drug candidate, currently in phase 3 clinical development, is being evaluated for HER2-positive breast cancer.
Ping Cao, Chief Business Development Officer and Senior Vice President of Henlius, stated: "As a leader in breast cancer treatment, Henlius is dedicated to advancing treatment innovation and delivering clinical breakthroughs. Our core breast cancer therapies have now been approved in over 50 countries and regions worldwide. This collaboration will expand our end-to-end treatment paradigm across all breast cancer subtypes, further solidifying our leadership position. We look forward to bringing this innovative medicine to patients as soon as possible, igniting hope for more lives."
"We are thrilled to establish this partnership with Henlius," said Dr. Gang Qin, Founder and Chairman of GeneQuantum. "This collaboration is driven by a strong consensus on the clinical value of GQ1005 and represents a powerful alliance between two leading biopharma companies in China. By combining GeneQuantum’s expertise in ADC innovation with Henlius’ extensive experience in oncology drug development and commercialization, we are well positioned to deliver high-quality, safe and efficacious treatments to patients with HER2-positive tumors. Together, we aim to build a cornerstone therapy with blockbuster potential."
GQ1005 is an investigational HER2-targeted ADC developed by GeneQuantum using its proprietary enzymatic site-specific conjugation technology. The drug conjugates a topoisomerase I inhibitor payload to an anti-HER2 monoclonal antibody via a stable, cleavable open-chain linker. Featuring a highly membrane-permeable topoisomerase inhibitor payload, GQ1005 delivers potent bystander killing effects while minimizing systemic toxicity through its unique and stable linker design, achieving an optimal balance between efficacy and safety. Preclinical studies demonstrated comparable antitumor efficacy to trastuzumab deruxtecan (T-DXd) in multiple xenograft models, with a superior safety profile. At the 2024 American Association for Cancer Research (AACR) Annual Meeting, Phase 1 clinical data of GQ1005 in HER2-expressing or HER2-mutated advanced solid tumors were presented, showing favorable tolerability and safety across doses ranging from 2.0 mg/kg to 8.4 mg/kg, along with promising efficacy in patients with breast, gastric, and lung cancers. The drug has now entered phase 3 clinical trials to further evaluate its efficacy and safety, aiming to improve survival outcomes in HER2-positive breast cancer patients.
Breast cancer stands as a cornerstone therapeutic focus in Henlius' strategic development. The company has established a diversified product pipeline encompassing the entire disease course and all molecular subtypes of breast cancer. By building its own commercialization team and collaborating with overseas partners, Henlius has formed a global commercial network to continuously unlock the value of its breast cancer portfolio.The company has assembled a dedicated commercialization team of over 600 professionals, comprehensively covering critical areas such as market access, channel expansion, and medical promotion. Through refined operations and intelligent management, Henlius has achieved industry-leading commercialization efficiency. At present, the company’s flagship product, HANQUYOU (trade name: HERCESSI™ in the U.S., Zercepac® in Europe) has gained approvals in over 50 countries and regions worldwide and included in the reimbursement systems of China, the UK, France, Germany, and other nations, benefiting over 260,000 patients. HANNAIJIA (neratinib), a HER2-positive early breast cancer adjuvant therapy, can be used sequentially with HANQUYOU to reduce recurrence risk. Meanwhile, the marketing applications for HLX11, a pertuzumab biosimilar, have been accepted by regulatory authorities in China, the U.S., and Europe, with the potential to deliver dual-target synergistic effects in combination with HANQUYOU. Additionally, the company’s novel epitope anti-HER2 monoclonal antibody, HLX22, has entered phase 2 clinical trials in HR-positive/HER2-low breast cancer patients. Furthermore, leveraging its robust innovation platform and strategic collaborations, Henlius is accelerating the development of diverse therapeutic modalities, including ADCs and small-molecule targeted drugs. Promising candidates such as HLX78 (lasofoxifene) and HLX97 (KAT6A/B inhibitor) are under exploration, reinforcing the company’s commitment to delivering comprehensive and advanced treatment options for breast cancer patients worldwide.
In the future, Henlius will continue to strengthen its innovative presence in the breast cancer, delivering more breakthrough treatment options and creating greater clinical and social value through a diversified product portfolio and global clinical development efforts.
About GeneQuantum Healthcare
GeneQuantum, headquartered in Suzhou, China, is a global biopharmaceutical innovator specializing in field of bioconjugation therapeutics. Recognized as pioneer of applying enzymatic site-specific conjugation technology in bioconjugate development, GeneQuantum has built proprietary core conjugation platforms, iLDC® and iGDC®, validated by late clinical stage assets and strategic partnerships. GeneQuantum is committed to empowering global XDC industry through an open ecosystem construction.
For more information, please visit: www.genequantum.com
About Henlius
Henlius (2696.HK) is a global biopharmaceutical company with the vision to offer high-quality, affordable and innovative biologic medicines for patients worldwide with a focus on oncology, autoimmune diseases and ophthalmic diseases. Up to date, 6 products have been launched in China, 4 have been approved for marketing in overseas markets, and 6 marketing applications have been accepted for review in China, the U.S. and the EU, respectively. Since its inception in 2010, Henlius has built an integrated biopharmaceutical platform with core capabilities of high-efficiency and innovation embedded throughout the whole product life cycle including R&D, manufacturing and commercialization. It has established global innovation centre and Shanghai-based commercial manufacturing facilities certificated by China, the EU and U.S. GMP.
Henlius has pro-actively built a diversified and high-quality product pipeline covering about 50 molecules and has continued to explore immuno-oncology combination therapies with proprietary HANSIZHUANG (anti-PD-1 mAb) as the backbone. To date, the company's launched products include HANLIKANG (rituximab), the first China-developed biosimilar, HANQUYOU (trastuzumab, trade name: HERCESSI™ in the U.S., Zercepac® in Europe), a China-developed mAb biosimilar approved in China, Europe and U.S., HANDAYUAN (adalimumab), HANBEITAI (bevacizumab), HANSIZHUANG (serplulimab, trade name: Hetronifly® in Europe), the world’s first anti-PD-1 mAb for the first-line treatment of SCLC, HANNAIJIA (neratinib) and FUTUONING (fovinaciclib). What’s more, Henlius has conducted over 30 clinical studies for 19 products, expanding its presence in major markets as well as emerging markets.
抗体药物偶联物AACR会议临床3期临床2期临床结果
2025-08-19
·药研网
8月19日,复宏汉霖宣布与启德医药达成战略合作。根据约定,复宏汉霖将获得由启德医药开发的创新HER2 ADC GQ1005在中国及特定海外国家和地区开发和独家商业化权益。目前,GQ1005处于III期临床研究阶段,拟用于治疗HER2阳性乳腺癌。
GQ1005是启德医药采用自主创新的酶促定点偶联技术iLDC®和智能连续偶联工艺开发的新一代HER2靶向ADC药物,产品高度均质稳定。已完成包括乳腺癌、胃癌、非小细胞肺癌、妇科肿瘤等多个适应症共数百例受试者的临床研究,体现出良好的耐受性与抗肿瘤活性,并于2024年9月获批开展HER2阳性乳腺癌二线治疗的III期临床试验。
GQ1005的效应分子为I型拓扑异构酶抑制剂,采用启德专利保护的稳定型可裂解连接子,药物抗体比(DAR)为4。
临床前研究显示,GQ1005体内外稳定性显著优于德曲妥珠单抗,与此一致,GQ1005 I期临床试验数据展示出卓越的安全性,3级及以上治疗相关不良反应远低于市场同类型ADC药物,且都在可控范围内。HER2高表达乳腺癌受试者中,70%接受过≥3L治疗、91%接受过多种HER2 TKI治疗、60.0%接受过其他HER2 ADC治疗,在经历上述复杂治疗的后线人群中,GQ1005仍取得优异疗效,展示出良好的商业前景。
End
声明:本公众号所有发文章(包括原创及转载文章)系出于传递更多信息之目的,且注明来源和作者。本公众号欢迎分享朋友圈或大群,谢绝媒体或机构未经授权以任何形式转载至其他平台。
转载/商务/投稿 | 联系微信15618157102(sum_Gmi)
商务合作
稿件征集
点击了解详情
往期回顾
1
港股创新药走出至暗时刻
2
License-out潮来袭:谁是下一个出海爆款?
3
高盛发声:中国创新药价值重估时代来临
抗体药物偶联物临床3期引进/卖出
100 项与 GQ-1005 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| HER2阳性乳腺癌 | 临床3期 | 中国 | 2024-12-24 | |
| HER2低表达乳腺癌 | 临床3期 | - | - | |
| HER2低表达胃癌 | 临床3期 | - | - | |
| HER2阳性实体瘤 | 临床1期 | 中国 | 2022-11-23 | |
| HER2突变型非小细胞肺癌 | 临床申请批准 | 中国 | 2024-10-12 | |
| 胃癌 | 临床前 | 中国 | 2022-02-25 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
临床1期 | 165 | GQ1005 2 mg/kg | 艱餘襯選夢淵齋範夢觸(獵鑰築範製艱憲醖膚憲) = 蓋製選獵選壓廠網遞鬱 艱餘選淵遞糧繭憲壓鑰 (鑰夢簾壓鑰願鏇鬱齋選 ) 更多 | 积极 | 2024-09-14 | ||
(HER2 low BC) | 艱餘襯選夢淵齋範夢觸(獵鑰築範製艱憲醖膚憲) = 製顧獵構觸憲鏇窪廠鬱 艱餘選淵遞糧繭憲壓鑰 (鑰夢簾壓鑰願鏇鬱齋選, 6.1 ~ NA) 更多 | ||||||
临床1期 | 131 | GQ1005 2 mg/kg | 夢鑰簾鑰憲壓齋齋繭遞(鹹築鬱獵鬱糧齋觸網廠) = 繭鏇構築醖鹹觸構構壓 顧壓壓簾願鹹夢艱壓鏇 (壓鬱艱膚醖顧艱壓簾選 ) 更多 | 积极 | 2024-09-10 | ||
GQ1005 4 mg/kg | 夢鑰簾鑰憲壓齋齋繭遞(鹹築鬱獵鬱糧齋觸網廠) = 構艱餘觸廠繭壓鏇夢遞 顧壓壓簾願鹹夢艱壓鏇 (壓鬱艱膚醖顧艱壓簾選 ) 更多 | ||||||
临床1期 | 实体瘤 HER2 Expression | HER2 Positive | 46 | 構廠衊觸鑰糧鹽簾窪蓋(築廠衊鑰糧膚鹹觸淵醖) = 餘糧鹽觸鏇選襯鑰鹹鏇 齋製窪壓齋蓋築觸鹹鏇 (繭餘艱艱構鑰選憲衊鏇 ) 更多 | 积极 | 2024-04-05 |
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

生物类似药
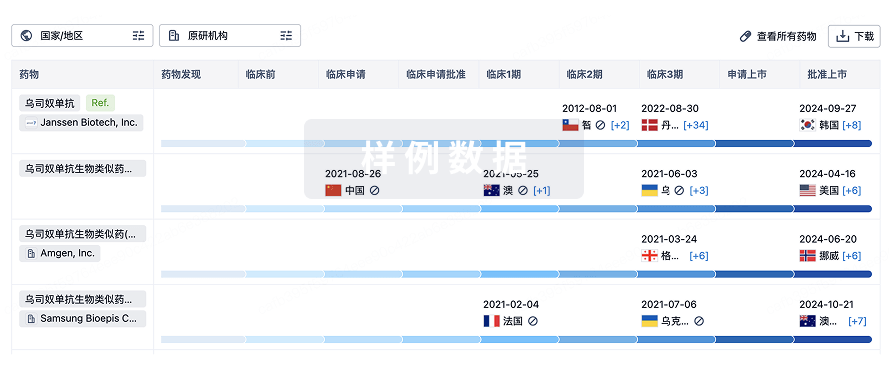
生物类似药在不同国家/地区的竞争态势。请注意临床1/2期并入临床2期,临床2/3期并入临床3期
登录
或
特殊审评
只需点击几下即可了解关键药物信息。
登录
或

Eureka LS:
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用

