预约演示
更新于:2025-05-07
Chlamydia trachomatis infection
沙眼衣原体感染
更新于:2025-05-07
基本信息
别名 CT - Chlamydia trachomatis infection、Chlamydia trachomatis infection、Chlamydia trachomatis infection (disorder) + [4] |
简介- |
关联
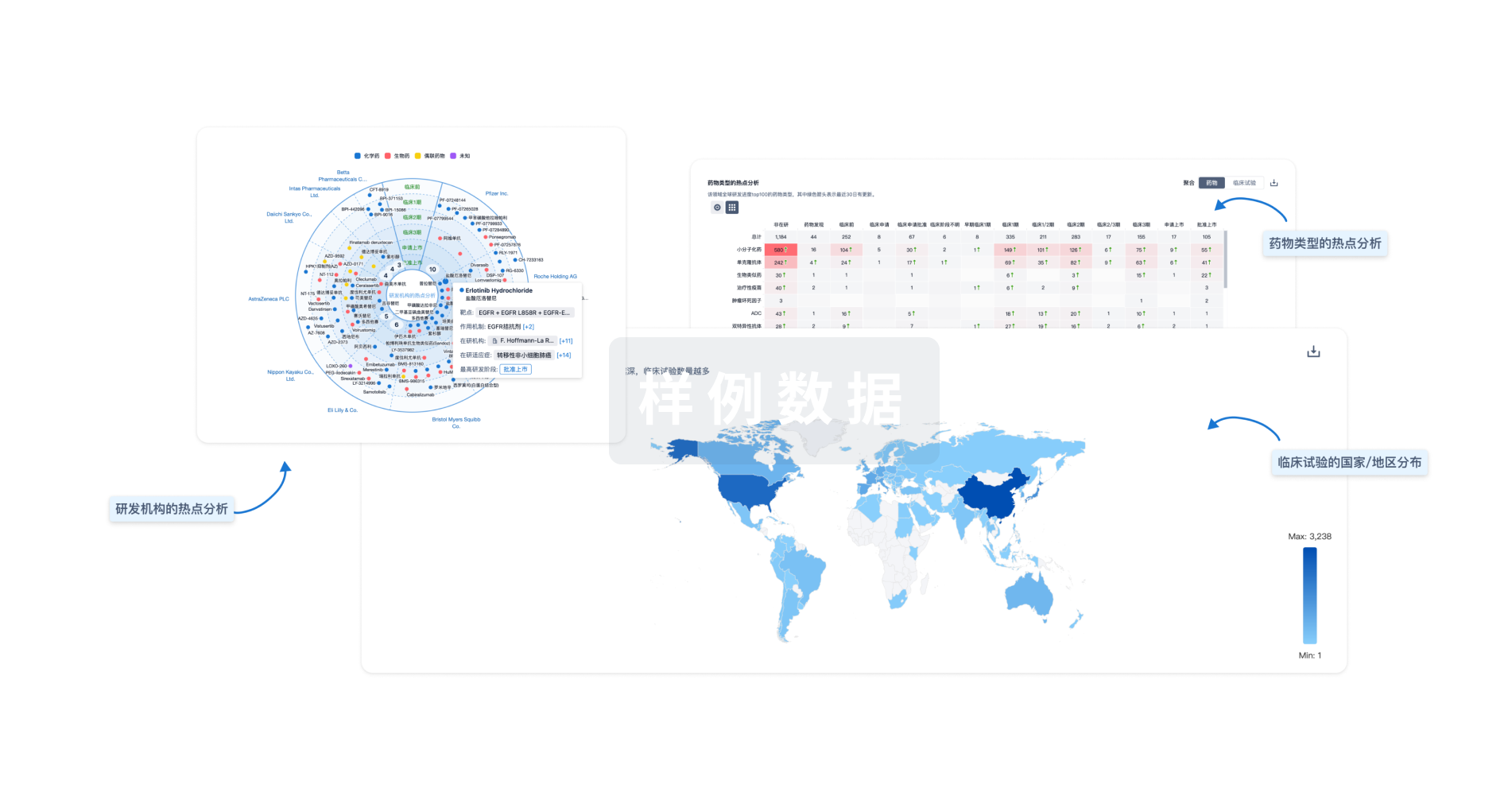
11
项与 沙眼衣原体感染 相关的药物靶点- |
作用机制 Inflammation mediators调节剂 |
在研机构 |
在研适应症 |
最高研发阶段批准上市 |
首次获批国家/地区 美国 |
首次获批日期2020-05-22 |
靶点 |
作用机制 RNAP抑制剂 |
在研机构 |
原研机构 |
最高研发阶段临床3期 |
首次获批国家/地区- |
首次获批日期1800-01-20 |
靶点- |
作用机制- |
在研机构 |
原研机构 |
非在研适应症- |
最高研发阶段临床2期 |
首次获批国家/地区- |
首次获批日期1800-01-20 |
88
项与 沙眼衣原体感染 相关的临床试验ChiCTR2500098675
Digital relay screening intervention model promotes self-sampling of gonorrhea and chlamydia among men who have sex with men in Shandong and Guangdong provinces: a pilot trial
开始日期2025-05-15 |
申办/合作机构- |
NCT06891417
A Phase 1/2, Randomized, Placebo-controlled, Multi-arm, Dose-finding Study to Evaluate the Safety, Immunogenicity, and Efficacy of a Chlamydia Trachomatis mRNA Vaccine Candidate in Adults Aged 18 to 29 Years
The purpose of this study is to evaluate the safety, efficacy, and immunogenicity of different dose levels (low, medium, and high) of Chlamydia messenger ribonucleic acid (mRNA) Vaccine candidate in adult participants aged 18 to 29 years.
This study will consist of 3 Sentinel Cohorts and a Main Cohort, with the Sentinel Cohorts assessing the safety of the different dose levels in a stepwise manner.
All participants will be followed up to 12 months after the last study intervention administration. Thus, the expected duration of the participant's involvement will be 18 months.
This study will consist of 3 Sentinel Cohorts and a Main Cohort, with the Sentinel Cohorts assessing the safety of the different dose levels in a stepwise manner.
All participants will be followed up to 12 months after the last study intervention administration. Thus, the expected duration of the participant's involvement will be 18 months.
开始日期2025-03-27 |
申办/合作机构 |
CTR20250153
罗红霉素分散片在健康受试者中单中心、随机、开放、单剂量、两制剂、两周期、两序列交叉设计空腹给药人体生物等效性研究
本试验旨在研究单次空腹口服哈药集团制药六厂研制、生产的罗红霉素分散片(150 mg)的药代动力学特征;以Opella Healthcare International SAS生产的罗红霉素片(罗力得®,150 mg)为参比制剂,比较两制剂中药动学参数Cmax、AUC0-t、AUC0-∞,评价两制剂的人体生物等效性。
开始日期2025-01-13 |
申办/合作机构 |
100 项与 沙眼衣原体感染 相关的临床结果
登录后查看更多信息
100 项与 沙眼衣原体感染 相关的转化医学
登录后查看更多信息
0 项与 沙眼衣原体感染 相关的专利(医药)
登录后查看更多信息
4,463
项与 沙眼衣原体感染 相关的文献(医药)2025-05-01·Journal of Adolescent Health
Identifying Provider Attitudes, Practices, and Barriers to Extra-Genital Testing for Neisseria Gonorrheae and Chlamydia Trachomatis Infections Among Adolescents and Young Adults
Article
作者: Wiemann, Constance M ; Babayev, Roman ; Zucker, Jason ; Hergenroeder, Albert ; Raphael, Meghna ; Ricondo, Stefani ; DeCuir, James ; Richards, Paul
2025-05-01·Sexually Transmitted Diseases
The Impacts and Consequences of Sexually Transmitted Infections in the United States
Review
作者: Allan-Blitz, Lao-Tzu ; Klausner, Jeffrey D.
2025-04-08·Infection and Immunity
Type I IFNs contribute to upregulation of PD-L1 during
Chlamydia trachomatis
infection
Article
作者: Starnbach, Michael N. ; Reinhold-Larsson, Nicole V.
19
项与 沙眼衣原体感染 相关的新闻(医药)2025-04-01
免疫新谈2025年3月25日 – 美国食品药品监督管理局(FDA)已授予赛诺菲预防衣原体感染的mRNA候选疫苗快速通道资格认定。这一决定是基于该候选疫苗在应对严重健康问题和解决未被满足的公共卫生需求方面的巨大潜力。该衣原体候选疫苗拟用于预防由沙眼衣原体引起的原发性生殖道感染和再感染。在临床前研究取得积极结果后,赛诺菲正计划开展一项1/2期随机临床研究,评估该候选疫苗在18至29岁成年人中的免疫原性和安全性。这一研究预计于未来几天内启动。Jean-François Toussaint赛诺菲疫苗研发全球负责人目前全球有数百万未被确诊的衣原体感染者,其中包括如果不及时治疗可能会导致严重的长期健康问题的无症状感染者。而抗生素治疗在遏制感染率上升方面作用有限。通过这一产品,我们期待让衣原体感染变为一种可通过接种疫苗预防的疾病。衣原体感染是全球最常见的细菌性性传播疾病,主要由沙眼衣原体引起。2020年,全球15至49岁人群中报告了1.29亿例衣原体感染病例,其中青少年和年轻成年人感染率最高。(图为衣原体3D模型)尽管抗生素可用于治疗已经确诊的衣原体感染,但衣原体感染者中无症状感染者的比例超过80%,这意味着感染却未被发现的风险很高,从而导致未治疗病例和意外传播。据山东一项横断面研究显示,在普通人群中,男性和女性的感染率分别为2.7%和2.3%,该感染率远高于全国报告发病率1,提示我国可能存在大量未被发现的衣原体感染。2此外,现有的预防措施未能有效控制感染率的上升,这更凸显了这一公共卫生领域对疫苗的迫切需求。衣原体候选疫苗的研发是赛诺菲与昆士兰政府、格里菲斯大学和昆士兰大学合作设立的“转化科学中心”的重要项目。该中心致力于促成澳大利亚昆士兰的顶尖研究人员与赛诺菲法国和美国的科学家间的沟通合作。参考文献:1.The incidence of genital chlamydial in China is 55.32/100,000,山东数据2.7% 或2.3%是全国发病率(55.32/100,000)的50倍。Yue, Xiao-Li et al., Epidemiology of Genital Chlamydial Infection in China in 2019. International Journal of Dermatology and Venereology 3(2):p 86-90, June 2020. | DOI: 10.1097/JD9.00000000000000992.Huai, P., Li, F., Li, Z. et al. Prevalence, risk factors, and medical costs of Chlamydia trachomatis infections in Shandong Province, China: a population-based, cross-sectional study. BMC Infect Dis 18, 534 (2018). https://doi-org.libproxy1.nus.edu.sg/10.1186/s12879-018-3432-y 中华医学会皮肤性病学分会,中国疾病预防控制中心性病控制中心,中国医师协会皮肤科医师分会,等.中国沙眼衣原体泌尿生殖道感染临床诊疗指南(2024)[J].中华皮肤科杂志, 2024, 57(03):193-200.DOI:10.35541/cjd.20230712. 向上滑动了解更多关于赛诺菲赛诺菲是一家全球领先的创新医药健康企业。我们的使命是追寻科学奇迹,焕发生命光彩。赛诺菲的足迹遍及全球100多个国家,致力于变革医疗实践,将不可能变为可能。我们为世界各地的人们提供潜在改变生活的医疗健康解决方案以及预防可致命疾病的疫苗,同时将可持续和社会责任置于我们雄伟战略的核心。关于赛诺菲中国赛诺菲是一家全球领先的创新医药健康企业,以“追寻科学奇迹,焕发生命光彩”为使命。作为改革开放后首批进入中国的跨国企业之一,赛诺菲1982年便在中国建立了办公室,目前拥有12处多元模式的办公室,3家生产基地和4大研发基地,多元化业务覆盖制药、人用疫苗和消费者保健。赛诺菲与中国同心同行,致力于将创新药品和疫苗加速引进中国,不断变革医疗实践,造福更多中国百姓,也为合作伙伴、社区和员工创造更美好的生活。如需了解更多信息,请访问www.sanofi.cn ,或关注“赛诺菲中国”微信公众号。 赛诺菲前瞻性声明 本新闻稿包含199年修订的《私人证券诉讼改革法案》中定义的前瞻性声明。前瞻性声明并非对历史事实的陈述。这些声明包括这些声明包括预测和估计以及其基础假设,涉及未来财务结果、事件、运营、服务、产品开发和潜力的计划、目标、意图和期望的陈述,以及与未来业绩相关的陈述。通常可以利用诸如“期望”、“预期”、“相信”、“打算”、“估计”、“计划”等词语,以及类似表达作为判定前瞻性声明的依据。尽管赛诺菲管理层认为该篇前瞻性声明中所反映的预期具有合理性,投资者仍需注意这些前瞻性信息和声明受制于诸多风险和不确定性因素,其中许多难以预测且通常不被赛诺菲所控制,这可能导致实际结果和发展与前瞻性信息和陈述中所表达、暗示或预测的信息存在重大差异。这些风险和不确定因素包括但不限于:研究和开发中固有的不确定因素,未来的临床数据和分析,包括产品上市后所获取的数据和所进行的分析,美国食品和药品监督管理局(FDA)或欧洲药品管理局(EMA)等监管机构对任何药物、器械或生物制品申请是否以及何时批准的决定,以及对标签和其他可能影响该类候选产品可得性或商业潜力的事项的决定,批准的候选产品可能商业上不成功的事实,替代性疗法的未来批准和商业成功,赛诺菲从外部增长机会中受益,完成相关交易和/或获得监管批准的能力,续与知识产权相关的待决或未来诉讼和这种诉讼的最终结果有关的风险,汇率和利率的趋势,以及波动的经济和市场条件,成本控制措施及其后续变化,以及流行病或其他全球危机可能给我们、客户、供应商和其他业务合作伙伴以及任何一方的财务状况带来的影响,也包括对我们的员工和全球经济带来的影响。这些风险和不确定性也包括赛诺菲在公开呈报给美国证券交易委员会(SEC)和法国金融市场管理局(AMF)的报告中已作讨论或明确的部分,其中包括列于表20-F的赛诺菲年度报告(截止日期2023年12月31日)中的“风险因素”和“前瞻性声明警示”。除非存在可适用的法律规定,赛诺菲不承担更新和修改任何前瞻性信息和陈述的义务。
疫苗临床研究信使RNA快速通道
2025-04-01
TUESDAY, April 1, 2025 -- The U.S. Food and Drug Administration granted marketing authorization for the first home-based, nonprescription diagnostic test for chlamydia, gonorrhea, and trichomoniasis in women, the agency announced Friday.
Women with or without symptoms can use the Visby Medical Women's Sexual Health Test to test for these three sexually transmitted infections (STIs). Results are delivered in about 30 minutes.
The single-use, at-home test includes a self-collected vaginal swab and a powered testing device that provides secure communication to the Visby Medical App. Once complete, the test results are displayed on the app.
This approval follows authorization of the first at-home
syphilis test
last year and authorization of the first
diagnostic test
for chlamydia and gonorrhea with at-home sample collection in 2023 -- the first FDA-authorized test with at-home sample collection for an STI other than HIV.
The approval was based on data showing the Visby Medical Women's Sexual Health Test correctly identified 98.8 and 97.2 percent of negative and positive
Chlamydia trachomatis
samples; 99.1 and 100 percent of negative and positive
Neisseria vaginalis
samples; and 98.5 and 97.8 percent of negative and positive
Trichomonas vaginalis
samples, respectively.
The FDA notes that women with positive results should consult with a physician. Those with symptoms, recent STI exposure, or other concerns should undergo additional testing. As with other tests, the main risks of using the Visby test are the possibility of false-negative results, leading to delays in treatment, or false-positive results, leading to unnecessary treatment.
"Expanding access to tests for sexually transmitted infections is an important step toward earlier and increased diagnosis, which can result in increased treatment and reduced spread of infection," Courtney Lias, Ph.D., director of the Office of In Vitro Diagnostic Devices in the FDA Center for Devices and Radiological Health, said in an agency news release.
More Information
Whatever your topic of interest,
subscribe to our newsletters
to get the best of Drugs.com in your inbox.
诊断试剂上市批准临床结果
2025-03-28
·美通社
衣原体感染可能导致女性盆腔炎,进而引发妊娠并发症或不孕。
一项评估该候选疫苗的免疫原性和安全性的1/2期临床研究将于未来几天内启动。
巴黎
2025年3月28日
/美通社/ -- 2025年3月25日,美国食品药品监督管理局(FDA)已授予赛诺菲预防衣原体感染的mRNA候选疫苗快速通道资格认定。这一决定是基于该候选疫苗在应对严重健康问题和解决未被满足的公共卫生需求方面的巨大潜力。
该衣原体候选疫苗拟用于预防由沙眼衣原体引起的原发性生殖道感染和再感染。在临床前研究取得积极结果后,赛诺菲正计划开展一项1/2期随机临床研究,评估该候选疫苗在18至29岁成年人中的免疫原性和安全性。这一研究预计于未来几天内启动。
赛诺菲疫苗研发全球负责人
Jean-François Toussaint
表示
:"
目前
全球
有数百万
未被确诊的
衣原体
感染者,其中
包括如果不及时治疗可能会导致严重的长期健康
问题的
无症状感染
者
。
而
抗生素
治疗在
遏制
感染率上升
方面作用有限
。
通过这一产品,
我们
期待
让
衣原体
感染
变
为一种可通过
接种
疫苗预防的疾病。"
衣原体感染是全球最常见的细菌性性传播疾病,主要由沙眼衣原体引起。2020年,全球15至49岁人群中报告了1.29亿例衣原体感染病例,其中青少年和年轻成年人感染率最高。
尽管抗生素可用于治疗已经确诊的衣原体感染,但衣原体感染者中无症状感染者的比例超过80%,这意味着感染却未被发现的风险很高,从而导致未治疗病例和意外传播。据山东一项横断面研究显示,在普通人群中,男性和女性的感染率分别为2.7%和2.3%,该感染率远高于全国报告发病率
[1]
,提示我国可能存在大量未被发现的衣原体感染。
[2]
此外,现有的预防措施未能有效控制感染率的上升,这更凸显了这一公共卫生领域对疫苗的迫切需求。
衣原体候选疫苗的研发是赛诺菲与昆士兰政府、格里菲斯大学和昆士兰大学合作设立的"转化科学中心"的重要项目。该中心致力于促成澳大利亚昆士兰的顶尖研究人员与赛诺菲法国和美国的科学家间的沟通合作。
[1]
The incidence of genital chlamydial in China is 55.32/100,000,山东数据2.7% 或2.3%是全国发病率(55.32/100,000)的50倍。
Yue, Xiao-Li et al., Epidemiology of Genital Chlamydial Infection in China in 2019. International Journal of Dermatology and Venereology 3(2):p 86-90, June 2020. | DOI: 10.1097/JD9.0000000000000099
[2]
Huai, P., Li, F., Li, Z.
et al.
Prevalence, risk factors, and medical costs of
Chlamydia trachomatis
infections in Shandong Province, China: a population-based, cross-sectional study.
BMC Infect Dis
18
, 534 (2018). https://doi-org.libproxy1.nus.edu.sg/10.1186/s12879-018-3432-y 中华医学会皮肤性病学分会,中国疾病预防控制中心性病控制中心,中国医师协会皮肤科医师分会,等.中国沙眼衣原体泌尿生殖道感染临床诊疗指南(2024)[J].中华皮肤科杂志, 2024, 57(03):193-200.DOI:10.35541/cjd.20230712.
关于赛诺菲中国
赛诺菲是一家全球领先的创新医药健康企业,以"追寻科学奇迹,焕发生命光彩"为使命。作为改革开放后首批进入中国的跨国企业之一,赛诺菲于1982年便在中国建立了办公室,目前拥有12处多元模式的办公室,3家生产基地和4大研发基地,多元化业务覆盖制药、人用疫苗和消费者保健。赛诺菲与中国同心同行,致力于将创新药品和疫苗加速引进中国,不断变革医疗实践,造福更多中国百姓,也为合作伙伴、社区和员工创造更美好的生活。
如需了解更多信息,请访问
www.sanofi.cn ,或关注"赛诺菲中国"微信公众号。
关于赛诺菲
赛诺菲是一家全球领先的创新医药健康企业。我们的使命是追寻科学奇迹,焕发生命光彩。赛诺菲的足迹遍及全球100多个国家,致力于变革医疗实践,将不可能变为可能。我们为世界各地的人们提供潜在改变生活的医疗健康解决方案以及预防可致命疾病的疫苗,同时将可持续和社会责任置于我们雄伟战略的核心。
赛诺菲前瞻性声明
本新闻稿包含
1995
年修订的《私人证券诉讼改革法案》中定义的前瞻性声明。前瞻性声明并非对历史事实的陈述。这些声明包括
这些声明包括预测和估计以及其基础假设,涉及未来财务结果、事件、运营、服务、产品开发和潜力的计划、目标、意图和期望的陈述,以及与未来业绩相关的陈述。
通常可以利用诸如"期望"、"预期"、"相信"、"打算"、"估计"、"计划"等词语,以及类似表达作为判定前瞻性声明的依据。尽管赛诺菲管理层认为该篇前瞻性声明中所反映的预期具有合理性,投资者仍需注意这些前瞻性信息和声明受制于诸多风险和不确定性因素,其中许多难以预测且通常不被赛诺菲所控制,这可能导致实际结果和发展与前瞻性信息和陈述中所表达、暗示或预测的信息存在重大差异。这些风险和不确定因素包括但不限于:研究和开发中固有的不确定因素,未来的临床数据和分析,包括产品上市后所获取的数据和所进行的分析,
美国食品和药品监督管理局(
FDA
)或欧洲药品管理局(
EMA
)等监管机构对任何药物、器械或生物制品申请是否以及何时批准的决定,以及对标签和其他可能影响该类候选产品可得性或商业潜力的事项的决定,批准的候选产品可能商业上不成功的事实,替代性疗法的未来批准和商业成功,赛诺菲从外部增长机会中受益,完成相关交易和
\/
或获得监管批准的能力,续
与知识产权相关的待决或未来诉讼和这种诉讼的最终结果有关的风险,
汇率和利率的趋势,
以及波动的经济和市场条件,
成本控制措施及其后续变化,以及流行病或其他全球危机可能
给我们、客户、供应商和其他业务合作伙伴以及任何一方的财务状况带来的影响,也包括对我们的员工和全球经济带来的影响。这些风险和不确定性也包括赛诺菲在公开呈报给美国证券交易委员会(
SEC
)和法国金融市场管理局(
AMF
)的报告中已作讨论或明确的部分,其中包括列于表
20-F
的赛诺菲年度报告(截止日期
2022
年
12
月
31
日)中的"风险因素"和"前瞻性声明
警示"。除非存在可适用的法律规定,赛诺菲不承担更新和修改任何前瞻性信息和陈述的义务。
疫苗快速通道临床研究信使RNA
分析
对领域进行一次全面的分析。
登录
或
生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用




