预约演示
更新于:2025-05-07
Cholesterol Ester Storage Disease
胆固醇酯贮积病
更新于:2025-05-07
基本信息
别名 CESD、CESD - Cholesterol ester storage disease、CHOLESTERYL ESTER STORAGE DISEASE + [11] |
简介 An autosomal recessive disorder caused by mutations in the gene for acid lipase (STEROL ESTERASE). It is characterized by the accumulation of neutral lipids, particularly CHOLESTEROL ESTERS in leukocytes, fibroblasts, and hepatocytes. |
关联
9
项与 胆固醇酯贮积病 相关的药物靶点 |
作用机制 LIPA调节剂 |
在研机构 |
最高研发阶段批准上市 |
首次获批国家/地区 欧盟 [+3] |
首次获批日期2015-08-28 |
靶点- |
作用机制- |
在研机构 |
原研机构 |
在研适应症 |
非在研适应症- |
最高研发阶段临床前 |
首次获批国家/地区- |
首次获批日期1800-01-20 |
40
项与 胆固醇酯贮积病 相关的临床试验NCT06650358
CLINICAL OUTCOMES of BILATERAL PSEUDOPHAKIC PATIENTS with a LIGHT ADJUSTABLE LENS+ (LAL)+ IMPLANTED in AT LEAST ONE EYE
The objective of this study is to collect data on bilateral pseudophakic patients implanted with the RxSight Light Adjustable Lens+ (LAL+) in at least one eye
开始日期2024-10-16 |
NCT06287658
The Effect of Kegel Exercise and Ba Duan Jin Applications on Quality of Life and Psychological Well-Being Applied to Premenopausal Women With Urinary Incontinence
This study aimed to determine the effects of Kegel exercise and Ba Duan Jin applications applied to premenopausal women with urinary incontinence on quality of life and psychological well-being. Study Group of the Research: Premenopausal women between the ages of 45-55 who come to the family health center with any complaint and have urinary incontinence. The research will be conducted as a randomized pre-test, post-test and control group intervention study design. The research will be conducted with women aged 45-55 with urinary incontinence who came for examination for any reason to a Family Health Center in Sinop between March 2024 and July 2024. According to the power analysis, the number of participants was calculated to be at least 54 when the type 1 error was taken as 0.05, the power was 0.95 and the effect size was medium (0.25) for the two-group design with two repeated measurements. To prevent possible data loss, the sample size was increased by 10% and the total number of participants was determined as 60. A total of 60 women, 30 experimental and 30 control, coming to the Family Health Center will be randomly included in the study. No information, Kegel exercise program and Ba Duan Jin applications will be given to the women in the experimental group, and no intervention will be given to the control group during the research process. Participants will be assigned to 2 groups: experimental and control. Those who come to FHC on odd days of the month will be included in the experimental group, and those who come on even days of the month will be included in the control group. Each group will be determined as 30 people. After the research is completed, the interventions applied to the experimental group will be applied to the women in the control group. Personal Information Form, Psychological Well-Being Scale and Incontinence Quality of Life Scale will be applied to women in the experimental and control groups as pre-test measurements. As a final test, the same measurements will be made to both the control group and the experimental group 16 weeks after the first measurement.
开始日期2024-04-01 |
申办/合作机构 |
NCT05687474
Universal Genomic Newborn Screening in the Wallonia-Brussels Federation: Baby Detect
Newborn screening (NBS) is a global initiative of systematic testing at birth to identify babies with pre-defined severe but treatable conditions. With a simple blood test, rare genetic conditions can be easily detected, and the early start of transformative treatment will help avoid severe disabilities and increase the quality of life.
Baby Detect Project is an innovative NBS program using a panel of target sequencing that aims to identify 126 treatable severe early onset genetic diseases at birth caused by 361 genes. The list of diseases has been established in close collaboration with the Paediatricians of the University Hospital in Liege. The investigators use dedicated dried blood spots collected between the first day and 28 days of life of babies, after a consent sign by parents.
Baby Detect Project is an innovative NBS program using a panel of target sequencing that aims to identify 126 treatable severe early onset genetic diseases at birth caused by 361 genes. The list of diseases has been established in close collaboration with the Paediatricians of the University Hospital in Liege. The investigators use dedicated dried blood spots collected between the first day and 28 days of life of babies, after a consent sign by parents.
开始日期2022-09-01 |
申办/合作机构 |
100 项与 胆固醇酯贮积病 相关的临床结果
登录后查看更多信息
100 项与 胆固醇酯贮积病 相关的转化医学
登录后查看更多信息
0 项与 胆固醇酯贮积病 相关的专利(医药)
登录后查看更多信息
754
项与 胆固醇酯贮积病 相关的文献(医药)2025-05-01·Journal of Inherited Metabolic Disease
Role of Biomarkers in Diagnosing Disease, Assessing the Severity and Progression of Disease, and Evaluating the Efficacy of Therapies
Review
作者: Schiffmann, Raphael
2025-03-01·Digestive Diseases and Sciences
Correction to: Pedigree Analysis of Nonclassical Cholesteryl Ester Storage Disease with Dominant Inheritance in a LIPA I378T Heterozygous Carrier
作者: Chen, Qian ; Gao, Mei-Zhu ; Luo, Jie-Wei ; Ruan, Dan-Dan ; Zhang, Li ; Fang, Zhu-Ting ; Lin, Fan ; Zheng, Xiao-Ling ; Lu, Shi-Yun ; Yu, Hong-Ping ; Liao, Li-Sheng ; Lin, Ai-Ping ; Chen, Meng-Shi ; Zhang, Jian-Hui ; Lin, Xin-Fu
2025-03-01·Clínica e Investigación en Arteriosclerosis
Extracellular vesicles in atherosclerosis: Current and forthcoming impact.
Review
作者: Civeira, Fernando ; Roncal, Carmen ; Cenarro, Ana ; Páramo, José A
20
项与 胆固醇酯贮积病 相关的新闻(医药)2024-10-30
·今日头条
【导读】
免疫力在2型糖尿病(T2D)和冠状动脉疾病(CAD)等心脏代谢疾病的药物开发方面,显示出潜力。团队进行了一项全转录组孟德尔随机化(MR)研究,以估计CD4 T细胞活化期间11,021个基因表达谱,对T2D和CAD发展的推定因果影响。
2024年10月26日,上海交通大学医学院附属瑞金医院陆洁莉团队、英国布里斯托大学等合作在期刊《Nature Communications》上发表了题为“Transcriptome-wide Mendelian randomization during CD4 T cell activation reveals immune-related drug targets for cardiometabolic diseases”的研究论文。在这项研究中,团队观察到162个改变T2D风险的基因和80个改变CAD风险的基因具有强大的MR和共定位证据,其中12%和16%分别证明了CD4 T细胞特异性。团队在69个基因-T2D对和34个基因-CAD对中,观察到了T细胞活化过程中的时间因果模式。团队确定了25个基因,
这些基因是临床研究中药物的靶标,
包括LIPA和GCK。
https://www-nature-com.libproxy1.nus.edu.sg/articles/s41467-024-53621-7
关于免疫调节药物
01
免疫力与1型糖尿病(T1D)治疗的革命有关。最近,美国食品和药品监督管理局(FDA)批准了一种靶向嵌合抗原受体(CAR)调节性T细胞的免疫治疗药物Teplizumab,用于治疗T1D2。免疫调节药物也显示出其在心血管疾病(CVD)治疗中的价值,包括白细胞介素6受体(IL6R)抑制剂的再利用。最近的研究,强调了免疫系统参与T2D的发展和β细胞功能的进行性恶化,这表明免疫和代谢之间的串扰,可能有助于T2D的发病机制。目前的免疫调节疗法,如IL-1β受体拮抗剂(IL-1βRa)和肿瘤坏死因子(TNF)α阻滞剂,在治疗T2D方面,已显示出临床疗效。
孟德尔随机化(MR)是一种经济高效的方法,已证明其在优先考虑药物靶标方面的价值。最近的研究提供了证据表明,靶向经过验证的遗传靶点的药物,更有可能在临床试验中取得成功。
在这项研究中,团队旨在利用在CD4 T细胞活化期间的5个时间点(0小时、lowly active[LA]、16小时、40小时和5天)获得的动态单细胞eQTL数据,来系统估计11,021个基因表达谱的推定因果效应。这有可能增强科学界对将免疫与心脏代谢疾病联系起来的潜在遗传机制的理解,并促进优先考虑T2D和CAD的潜在免疫介导药物靶点。
CD4 T细胞基因表达,对T2D和CAD的细胞类型特异性因果效应
02
与其他细胞类型相比,CD4 T细胞中这些已鉴定的基因表达之间的相关性如下:单核细胞为76%,NK细胞为86%,B细胞为78%,CD8 T细胞为94%。162个T2D基因中的23个(14%)和80个CAD基因中的8个(10%)对T细胞具有特异性,在其他免疫细胞类型中没有高表达。与随机选择的基因相比,通过遗传工具鉴定的T2D基因在T细胞中富集的可能性更高。这一观察结果在各种基因类别中是一致的,包括T细胞中表达、特异性表达和差异表达的基因。相比之下,几乎没有证据表明,CA基因在T细胞内表现出类似的富集。
16%的CAD基因和12%的T2D基因,仅在CD4 T细胞中具有eQTL。这些分析提供了进一步的证据,表明团队鉴定的基因在CD4 T细胞中高度表达;超过10%的鉴定T2D和CAD基因,可能表现出T细胞特异性。
细胞类型特异性分析结果。
确定T2D和CAD免疫相关药物靶点的优先级
03
在排名靠前的MR发现中,有4对具有MR和共定位证据的基因-疾病对,与正在进行临床试验评估的心脏代谢药物的相同靶点-适应症对相匹配。在这4个基因中,KHK、GCK和ERN1蛋白产物是抗糖尿病药物的靶点,而靶向GCK的药物在临床试验中仍然活跃。另一个基因LIPA是Sebelipase alfa(BLA:125561)的靶标,该酶已被美国食品和药品管理局(FDA)批准用于治疗溶酶体酸性脂肪酶缺乏症,溶酶体酸脂肪酶缺乏症可导致动脉粥样硬化。
此外,其中13个(52%)基因是目前正在临床开发中的药物的靶点,MR结果显示了推定的因果证据,支持将这些靶点重新用于T2D和CAD预防。例如,ERAP2是ERAP抑制剂(Grey Wolf Therapeutics)的靶基因,ERAP抑制剂是一种靶向抗原呈递中的ERAP蛋白酶的免疫疗法,目前正在进行癌症治疗(https://greywolftherapeutics.com)的临床前试验研究。它也被认为是治疗自身免疫性疾病的潜在靶点。MR结果显示强有力的遗传证据,支持抑制ERAP2表达水平在降低CAD风险中的因果作用(例如,0小时记忆CD4 T细胞中的ERAP2表达:OR=1.02,95% CI:1.01-1.03),这意味着ERAP抑制剂有机会重新定位,以预防CAD。
具有随机临床试验证据的基因详细信息
总结
04
1. 免疫介导基因表达与T2D/CAD的因果关系:
研究加强了对免疫介导基因表达与T2D/CAD之间潜在因果关系的理解,确定了一组与T2D风险相关的综合免疫介导基因。
2. CD4 T细胞与T2D的联系:
尽管CD4 T细胞与T2D有联系,但只有12%的基因对T2D表现出CD4 T细胞特异性作用,表明免疫细胞基因表达与T2D之间存在实质性相互作用。
3. 免疫失调在心脏代谢疾病中的作用:
免疫失调在心脏代谢疾病中的致病作用,免疫细胞如Tregs、Th17和B细胞在动脉粥样硬化进展过程中维持局部免疫力至关重要。
4. 免疫和代谢途径融合的研究潜力:
研究强调了免疫和代谢途径融合的潜力,为开发针对心脏代谢疾病的免疫调节药物铺平了道路。
参考资料:
1. Bluestone, J. A., Buckner, J. H. & Herold, K. C. Immunotherapy: building a bridge to a cure for type 1 diabetes. Science 373, 510–516 (2021).
2. Hirsch, J. S. FDA approves teplizumab: a milestone in type 1 diabetes. Lancet Diabetes Endocrinol. 11, 18 (2023).
【关于投稿】
转化医学网(360zhyx.com)是转化医学核心门户,旨在推动基础研究、临床诊疗和产业的发展,核心内容涵盖组学、检验、免疫、肿瘤、心血管、糖尿病等。如您有最新的研究内容发表,欢迎联系我们进行免费报道(公众号菜单栏-在线客服联系),我们的理念:内容创造价值,转化铸就未来!
转化医学网(360zhyx.com)发布的文章旨在介绍前沿医学研究进展,不能作为治疗方案使用;如需获得健康指导,请至正规医院就诊。
热门推荐活动 点击免费报名
🕓 上海|11月15日-16日
▶ 2024第一届中国类器官转化医学大会
🕓 上海|11月21日-24日
▶ 第七届上海国际肿瘤内科学论坛
🕓 全国|2024年12月-2025年03月
▶ 中国转化医学产业大会
🕓 上海|2025年02月28日-03月01日
▶ 第四届长三角单细胞组学技术应用论坛暨空间组学前沿论坛
点击对应文字 查看详情
免疫疗法细胞疗法临床研究临床结果信使RNA
2024-03-25
·同写意
据估计,全球约有3.5亿患者受罕见病影响,是艾滋病和癌症患者总数的两倍多。尽管当前罕见病研究取得重大进展,使得更深入理解其分子机制,同时也通过立法保证了监管和经济激励,从而推动罕见病特定疗法的开发。然而,将罕见病知识转化为疗法的速度远远落后于这些知识的生成速度。解决这一转化差距是一项多方面的挑战,其关键方面之一是选择最佳的治疗方式。来自比利时罕见病联盟的Erik Tambuyzer和Marco Prunotto研究团队在期刊《Nature Reviews Drug Discovery》上发表了题为“Therapies for rare diseases: therapeutic modalities, progress and challenges ahead”的文章,详细探讨了罕见病的主要治疗方式的技术基础和其适用性,包括小分子药物、抗体药物、蛋白质替代疗法、寡核苷酸疗法、基因和细胞疗法,以及药物再利用。对于每种治疗方式,作者比较了不同模式下的分子靶向机制(图1),描述了基于该平台的药物开发的临床进展,并分析了其作为罕见病疗法开发平台的优缺点。希望本文能为罕见病领域的药物发现提供入门介绍,并推动科学进步转化为新型疗法。图1 不同治疗模式的特点和监管批准数据(图片来源:Tambuyzer E et al, Nat Rev Drug Discov, 2020)1小分子药物小分子药物因多样的给药途径、可控的剂量、高稳定性、合成规模大以及相对较低的成本等优势,是罕见病药物研发中最成熟的药物平台。罕见病的小分子药物的发现通常来源于对模式细胞系的大规模筛选。在将候选小分子筛选转化用于临床疗法时,建立高通量筛选是局限性之一。近期,包括诱导多能干细胞(induced pluripotent stem cells, iPS)、基因编辑技术(CRISPR-Cas systems)和类器官(Organoids)在内的几项最新发展有效提升了筛选通量及效率。理论上,可以从患者的皮肤活检样本中建立iPS细胞,并分化为表达疾病表型特征的细胞类型。类器官3D培养更能模拟组织结构和功能,特别是当需要针对特定突变的药物时,它是筛选小分子药物的绝佳模型。模式生物筛选也正在成为重要的遗传和化学发现平台,特别是对于可能改变疾病表型的小分子药物。这些筛选还考虑到药物进入细胞中的摄取和毒性因素。CRISPR-Cas9基因编辑技术和转基因技术的出现使得可以引入特定的突变。同时,在几种模式生物中进行验证可以加速潜在疗法向临床的转化。对于囊性纤维化,根据对CFTR基因中潜在突变的了解,通过细胞筛选可获得治疗性小分子药物。当前FDA已批准两种抑制缺陷酶底物生物合成的小分子疗法(用于戈谢病的miglustat和eliglustat)和一种作为伴侣蛋白稳定并恢复突变酶功能的小分子疗法(用于法布里病的migalastat),还有其他候选药物正在进行临床试验,包括用于治疗法布里病的CNS穿透性化合物ibiglustat。在杜氏肌营养不良(duchenne muscular dystrophy, DMD)患者中,抗肌萎缩蛋白的编码基因存在终止密码子突变,小分子ataluren能够在小鼠模型中促进终止密码子通读,目前已在欧盟获得批准。小分子药物也可用于提升蛋白质水平,如促进肌营养相关蛋白表达,在动物模型中也可以减缓DMD。另外,几种处理下游效应(如炎症和纤维化)的小分子药物也在DMD临床试验中显示出疗效,可以联合使用。最后,目前正在开发用于LSDs的小分子蛋白稳态调节剂,可增强内源性细胞对应激的反应,并上调HSP70(heat shock protein 70)以促进蛋白质折叠。小分子药物在药物研发中仍处于前沿地位,其靶向广泛,生产成本合理且可大规模生产。对于罕见病而言,该平台有着丰富的科学、临床和监管经验。对于分子病因不明或有多重因素参与的罕见病,表型筛选也会对发现有效小分子起到正面作用。但同时,找到既具备好的药理学效应和药代动力学效应又能使脱靶效应低的小分子较为艰难,需要经历复杂的优化过程。当然,目前一些已被证明安全的其他药物可以应用于罕见病,随着对罕见病机理研究的深入,类似于靶向不同病因的联合疗法也将成为可能。2抗体药物抗体药物主要通过调节信号通路、募集细胞或蛋白质至特定部位、传递细胞毒素或中和及调节循环因子来发挥作用。单克隆抗体(monoclonal antibody, mAbs)由B淋巴细胞产生,能够在体液免疫反应中识别抗原。mAbs的两个关键特性是对特定抗原的特异性和这种特异性的持续性。针对第一批鼠源性mAbs具有免疫原性和较短的半衰期问题,研究者们设计了人-鼠混合mAbs、人源化小鼠mAbs或人源mAbs来开发有效mAbs治疗药物。另外,抗体工程的进步使得抗体可以以naked mAbs或更小的工程化抗原结合片段(antigen-binding fragments, Fab)的形式生产,帮助提高限定区域(如眼底)的药物浓度,降低全局生物活性,并降低毒性。工程技术还允许生产双特异性抗体(bispecific antibody, BsAb),相比mAbs更有优势,能够直接引导免疫系统靶向肿瘤细胞或同时阻断两个不同的靶点,迄今为止已有2种BsAb获批上市。抗体恒定区(fragment crystallizable, Fc)也可与其他非抗体相关蛋白结构域融合,作为单独的治疗性抗体,或将全长抗体与小分子融合,形成ADCs。Fc融合蛋白具有IgG的优势,能促进体内稳定性,实现特异性治疗,目前已有8种Fc融合蛋白被批准。ADCs利用mAbs的特异性,选择性地将细胞毒性药物递送至肿瘤细胞,从而减少对健康组织的损伤。目前只有少数ADCs被批准用于血液肿瘤,但该领域较为活跃,有超过50种ADCs正在进行临床试验。依库珠单抗(eculizumab)是一种靶向末端补体蛋白C5的mAb,10多年前首次被批准用于治疗阵发性睡眠性血红蛋白尿症(paroxysmal nocturnal hemoglobinuria,PNH),此后又被批准用于非典型溶血性尿毒综合征(atypical haemolytic uraemic syndrome)和重症肌无力(myasthenia gravis)。卡那单抗(canakinumab)最初是为类风湿性关节炎(rheumatoid arthritis)开发的一种靶向关键炎性细胞因子IL-1β的mAb,2009年被重新应用并批准用于治疗cryopyrin相关周期性综合征,该项目临床试验还为批准其用于治疗其他三种与IL-1β相关的罕见周期性发热综合征提供了依据。IL-1β也是利纳西普(rilonacept)的靶点,于2008年被批准用于治疗cryopyrin相关周期性综合征。目前已批准的两种BsAbs中,分别是血友病(hemophilia)治疗药物艾米珠单抗(Emicizumab)和获得性血栓性血小板减少性紫癜(acquired thrombotic thrombocytopenic purpura, ATTP)治疗药物卡普赛珠单抗(caplacizumab)。基于mAb的治疗平台具有高度特异性,脱靶毒性低,稳定性高,有助于降低给药频率。对于获能突变引发的罕见病,已经建立了合适的mAb疗法。然而,mAbs的大尺寸限制了其对组织和细胞的穿透,从而阻碍了对某些理论上理想靶点(如细胞内蛋白)的结合。由于需要在良好生产规范条件下大规模培养哺乳动物细胞并进行大量纯化步骤,因此mAbs的制造成本也非常高。此外,mAbs需要注射给药,因此在制剂阶段需要非常高的无菌标准,并且可能引发注射部位的不良反应。当前mAb平台研发部署较少,但高效、安全地识别和制造mAbs的能力正在“普及”,再加上在生产环节可实现更高的mAb滴度,这大大降低了商品成本。其次,mAbs的廉价生物仿制药进入市场可能有助于重新利用该抗体。3蛋白质替代疗法蛋白质替代疗法,长期以来一直是治疗特定蛋白质功能丧失相关的罕见病的重要基石。较为成功的方向是使用外源补充的酶来替代缺失或者功能缺陷的酶进行治疗—ERT疗法,ERT的研究主要靶向溶酶体酶相关的LSDs。应用于ERT的酶大部分来源于重组蛋白。ERTs通常使用哺乳动物细胞系生产,最常见的是中国仓鼠卵巢(CHO)细胞。与所有重组蛋白疗法一样,从生物反应器发酵液纯化制备的酶是复杂的,需要高度控制,以保持最终产品的生物活性和足够的产量。此外,制造参数(如生物反应器的规模)的变化可能导致最终产品出现特性差异,这可能会被监管机构认为有临床意义。迄今为止,全球已开发并批准了11种不同的重组ERTs,包括戈谢病、法布里病、Hurler-Scheie综合征(黏多糖贮积症Ⅰ型(MPSⅠ))、亨特综合征(MPS Ⅱ)、庞贝病、Maroteaux-Lamy综合征(MPS Ⅵ)、溶酶体酸性脂肪酶缺乏症(沃尔曼病)、巴顿病(神经蜡样质脂褐质沉积症2型)、Morquio A综合征(MPS ⅣA)以及最近的Sly综合征(MPS Ⅶ)和α-甘露糖苷贮积症。在LSDs领域,目前正在为圣菲利柏A综合征(MPS ⅢA)和圣菲利柏B综合征(MPS ⅢB)开发多种ERT。在LSDs领域之外,使用天然(人类和动物)酶的ERTs已被批准用于A1AT增强疗法和与腺苷脱氨酶(adenosine deaminase, ADA)缺乏相关的重症联合性免疫缺陷病(severe combined immunodefiency, SCID),重组ERTs也被批准用于低磷酸酯酶症(FDA和EMA)和苯丙酮尿症(FDA)。在病程的早期(即在重大不可逆器官损伤发生之前),替代酶能够以合适的剂量被输送到合适的组织和细胞,ERT就会非常有效。酶递送是受体介导并具有剂量依赖性,在戈谢病中,酶表面的甘露糖分子有助于酶进入相关细胞类型,即巨噬细胞。然而,对于其他LSDs,如黏多糖病、法布里病等,ERT的开发较为困难,因为病理性底物积聚发生在缺乏或表达低水平甘露糖受体的其他细胞类型中。此外,当酶需要被输送到血管系统服务较少的器官或组织时,需要较高的剂量才能达到正确的治疗效果。而静脉注射ERT对神经病变亚型的神经学表现无效,因为酶体积太大,无法穿过血脑屏障。因此,在一些LSDs试验中正在测试向CNS实现鞘内注射ERT。ERT疗法安全性高,很少有患者出现不良反应。但是可能会引起过敏,从而限制治疗的效果,并且对于受到不可逆器官损伤的重症患者,ERT治疗效果非常差。当前,ERT技术仍存在局限性,包括制造和纯化重组酶的成本,以及建立新产品制造能力所需的时间。4寡核苷酸疗法目前已经开发出几种靶向RNA的方法,其中研究最广泛的是ASOs和siRNAs,寡核苷酸疗法是通过序列特异的碱基配对与RNA靶点结合的合成核酸序列,从而以各种方式影响基因表达。ASOs是单链分子,并通过核糖核酸酶H引发其选择性降解,导致相应蛋白的表达下调。siRNAs属于内源性基因调控机制,为双链结构,通过整合修饰后的化学骨架以增强其药物特性。它们通常会与脂质纳米颗粒或N-乙酰半乳糖胺等载体结合实现肝脏递送。此外,单链RNA阻断剂通过与前体mRNA或mRNA杂交,也有助于基因功能障碍相关的罕见病的治疗,且能够恢复基因功能,而不是只像ASO和siRNA一样起到抑制作用。1998年,第一种获得FDA批准的ASO药物是用于治疗免疫受损患者(包括艾滋病患者)巨细胞病毒视网膜炎的福米韦森(fomivirsen)。虽然福米韦森后来已被撤市,但其他ASO药物也随之出现,例如米泊美生(ASO mipomersen)于2013年被批准用于治疗家族性纯合子高胆固醇血症。寡核苷酸疗法在罕见神经系统疾病中的应用或许最具前景,近年来获得数项开创性批准。其中Patisiran是一种与脂质相结合的siRNA,成为FDA批准的首个siRNA疗法,随后不久FDA批准了ASO药物Inotersen。这两种药物均是通过降解编码TTR的mRNA发挥作用。另外两种RNA阻断型寡核苷酸疗法Nusinersen和Eteplirsen也已被批准用于罕见的神经系统疾病。Nusinersen通过增加SMN2 mRNA转录本中的外显子7包含物发挥作用,从而生成SMN蛋白,已在美国和欧盟获批上市。Eteplirsen旨在校正翻译阅读框并生成缩短但仍具功能的抗肌萎缩蛋白,主要用于治疗DMD。然而Eteplirsen的效果一直存在争议。寡核苷酸疗法具有高度特异性,能够解决传统疗法无法触及的靶标,并且由于全身暴露有限而降低了毒性。这极大扩展了可选目标的数量和类型。由于大多数已知的罕见病是遗传性的,寡核苷酸疗法通过靶向RNA为降低罕见病所引发的较大的发病率和死亡率提供了关键机会。然而,寡核苷酸不易穿过血脑屏障,因此需要采取有创的给药方式(如鞘内或脑室内途径),这仍然是其在CNS疾病临床应用的最大障碍之一。尽管如此,近期获得监管机构批准的成功案例数量可能会推动其针对其他罕见病的研究和开发。例如,靶向亨廷顿蛋白(HTT)mRNA的ASO药物(RG6042),最近已进入临床Ⅲ期试验,希望这能成为针对这种神经退行性疾病的首个疾病修饰疗法。5基因与细胞治疗基因治疗在罕见病中的作用原理主要为两种,一种是表达所需蛋白以弥补特定蛋白质功能丧失,另一种是利用RNA干扰机制抑制基因表达。腺相关病毒(adeno-associated virus, AAV)载体和逆转录病毒(retrovirus)/慢病毒(lentivirus)载体是目前常用于罕见病临床研究的两种基因治疗平台。重组AAV载体可以插入小于5000个碱基的治疗性转基因以进行基因治疗。目前已鉴别出13种AAV血清型,不同的AAV血清型具有不同的组织趋向性。例如,AAV8已在三项临床试验中用于将基因递送至肝脏;AAV9已用于四项神经系统疾病的临床试验中。治疗用的AAV不会将基因整合进人体细胞,保证了安全性,但其会在细胞复制中丢失信息,因此只能用于非分裂(或分裂缓慢)细胞类型的基因治疗。AAV载体具有良好的安全性,在200多项临床试验中没有死亡或致癌记录。在人体内引起的最常见严重不良反应是肝转氨酶水平升高(表明肝损伤),与对AAV衣壳蛋白的免疫反应有关,通常可以通过类固醇治疗来控制。逆转录病毒包含单链RNA基因组,通过逆转录将基因整合到人类基因组中,实现基因组的永久修饰。慢病毒是逆转录病毒家族的一个属,不依赖有丝分裂进入细胞核,其感染效率更高且可以自我灭活,因此可用于递送基因至分裂或非分裂细胞中。根据最初的细胞来源,细胞疗法可分为患者特异性来源(通常是自体的)或已制备好的细胞来源这两类。可用的细胞类型包括T细胞、树突状细胞(dendritic cell, DC)、间充质基质细胞(mesenchymal stromal cell, MSC)、成纤维细胞、自然杀伤(natural killer,NK)细胞、神经干细胞、诱导多能干细胞(induced pluripotent stem cell, iPSC)等,通过靶细胞分离(选择、分选)、培养(扩增、激活)、洗涤、体积减少和其他配制组合的过程进一步修饰源细胞。离体修饰通常是通过使用病毒载体实现的。与体内基因组编辑相比,体外编辑的脱靶风险将大大降低。在临床应用方面,AAV疗法已被证实在脊髓性肌萎缩症(spinal muscular atrophy, SMA)、A型和B型血友病(hemophilia)、芳香族L-氨基酸脱羧酶缺乏症(aromatic L-amino acid decarboxylase deficiency, AADCd)等多种罕见病中有效,目前有8种AAV基因疗法已被批准上市(表1)。表1 全球获批上市的8款AAV基因治疗产品在单基因罕见疾病方面,AAV载体已经体现出临床优越性。相关研究表明,AAV载体可在非分裂细胞中持续多年地表达基因。但其生产制造的复杂性和成本远高于小分子,其他局限包括免疫应答导致的AAV转导细胞的丢失、缺乏用于其他相关组织和细胞类型的载体血清型、以及基因组的有限荷载。另外,基因组编辑完成后相关基因的长期表达可能会带来负面影响,但是其中的致癌问题可通过自灭活慢病毒载体解决。而细胞疗法的一个关键局限性是目前尚无法确定产品的特征以确保一致性,其确切作用机制可能也不清楚,因此提高了使用的成本。相较于小分子、抗体和蛋白质替代疗法,基因和细胞疗法尚处于早期发展阶段,然而,这种疗法可能是一次性治疗甚至可以治愈疾病,因此对罕见病具有深远的意义。— 总结 — 在详尽阐述了当前罕见病新药开发的主要技术平台之后,作者还简要概述了一些深刻影响此类药物研发环境的核心问题和关键因素,为全面理解罕见病药物研发领域提供了更为深入的视角。01孤儿药开发监管途径孤儿药开发的各种监管途径和经济激励措施,如今在美国、欧洲、日本和其他地方都已确立。为加快创新药的研发速度,解决重大未满足的医疗需求,已经出台了几项监管措施,包括美国的快速通道、加速审批、优先审查、突破性疗法认定和再生医学先进疗法认定,以及欧盟的有条件上市许可、特殊情况批准、加速评估、优先药物(PRIME)计划和先进疗法监管。对FDA和EMA的批准进行审查发现,这些项目更多地用于孤儿药,而非用于治疗非罕见病产品(表2)。表2 孤儿药的快速监管审查途径(图片来源:Tambuyzer E, Vandendriessche B, Austin CP, et. al, Nat Rev Drug Discov, 2020)02患者参与监管决策和孤儿药开发患者权益组织在孤儿药开发中将继续扩大其作为研究伙伴的角色,他们在确定临床终点、制定知情同意书、招募患者等方面提供专业知识,并参与监管机构会议,讨论研发活动以及医疗产品监管审查中的效益风险评估。同时在药物开发早期,患者的观点也极具价值。例如,在建立患者登记系统和生物样本库以及选择临床试验终点方面,这些在罕见病中往往未得到很好的确立。尽管已经努力选择适合目的的终点,但许多罕见病的异质性降低了候选药品的敏感性,再加上患者数量少,可能会导致假阴性。通过使用可以在临床试验前、中和后期实施的半结构化视频访谈,来捕捉患者及其看护者对变化的看法,可以防止这种假阴性。另外对于从事孤儿药开发的研究人员来说,与相关患者建立良好关系非常重要,并且应该尽早建立。03推动罕见病疗法的有效研究和开发罕见病患者群体多样,值得作为更高的公共卫生优先事项来对待。罕见病领域的所有利益相关者都需要协作,以弥合罕见病领域基础研究与治疗开发之间的巨大鸿沟。当前,对罕见病的高质量研究没有到达临床阶段就结束了。在美国和欧盟,尽管孤儿药的数量都在稳步增长(图2),但仅有约5%的孤儿药获批。此外,一些疾病领域也存在巨大的偏倚,超过1/3的孤儿药被批准用于肿瘤适应证(图3)。图2 罕见病在科学知识和治疗方法之间的巨大差距(图片来源:Tambuyzer E, Vandendriessche B, Austin CP, et. al, Nat Rev Drug Discov, 2020)图3 罕见病和孤儿药的关键因素(图片来源:Tambuyzer E, Vandendriessche B, Austin CP, et. al, Nat Rev Drug Discov, 2020)对于肿瘤以外的罕见病,在将现有基础科学转化为治疗方法方面存在一个重大缺口。标准技术平台和就绪性的评估机制可以增强患者组织的能力,并促进治疗开发的进展。将罕见病的基础研究转化为治疗方法已成为公共和慈善事业的首要任务,提供资金支持,并为患者群体提供咨询和支持服务,这将有助于降低早期研究结果的风险,让更多行业参与推动新疗法。此外,在全球合作中共享诊断数据,能够保护患者隐私,将有助于更轻松、更快速地诊断罕见病,并推动跨疾病分析。数据的统一可以减少重复工作和成本,提高效率,增加领域专业知识。传感器技术的日益普及将提供更多研究数据,如识别具有相似症状的不同的疾病可能共享的药物反应模式等。在建立登记系统以及临床和自然史研究时,也应咨询患者组,以确保考虑患者的观点。最后,通过对罕见病药物研发中使用的技术平台的概述,希望这篇文章将成为帮助基础科学转化为此类疾病治疗开发的宝贵资源。同时还希望,这将引发人们对资助和培养转化科学文化的兴趣,以减轻全球罕见疾病患者的痛苦。关于劲帆医药劲帆生物医药科技(武汉)有限公司简称“劲帆医药”,创立于2022年,提供一站式基因治疗药物CRO/CDMO服务。拥有全球领先的AAV规模化制备专利技术平台及cGMP级病毒载体生产车间,致力于推进基因治疗更有效,更安全,更经济,更可及,赋能客户,造福患者。关于同写意 同写意论坛是中国新药研发行业权威的多元化交流平台,二十年来共举办会议论坛百余期。“同写意新药英才俱乐部”基于同写意论坛而成立,早已成为众多新药英才的精神家园和中国新药思想的重要发源地之一。同写意在北京、苏州、深圳、成都设立多个管理中心负责同写意活动的运营。尊享多重企业/机构会员特权 ● 分享庞大新药生态圈资源库;● 同写意活动优享折扣;● 会员专属坐席及专家交流机会;● 同写意活动优先赞助权;● 机构品牌活动策划与全方位推广;● 秘书处一对一贴心服务。入会请联系同写意秘书处 同写意创新链盟机构 (上下滑动查看更多)森西赛智 | 汇芯生物 | 申科生物 | 方拓生物 | 东抗生物 | 科盛达 | 依利特 | 翊曼生物丨锐拓生物丨复百澳生物丨圆因生物丨普洛斯丨华润三九丨皓阳生物丨人福医药丨广生堂药业丨澳宗生物丨妙顺生物 | 荣捷生物丨行诚生物 | 宜联生物 | 生命资本 | 恒诺康丨益诺思 | 深圳细胞谷丨佰诺达生物 | 沃臻生物 | 金仪盛世 | 朗信生物 | 亦笙科技 | 中健云康 | 九州通 | 劲帆医药 | 沙砾生物 | 裕策生物 | 同立海源 | 药明生基 | 奥浦迈 | 原启生物 | 百力司康 | 宁丹新药 | 上海细胞治疗集团 | 滨会生物 | FTA | 派真生物 | 希济生物 | 优睿赛思 | 血霁生物 | 优睿生物 | 邦耀生物 | 华大基因 | 银诺生物 | 百林科医药 | 纳微科技 | 可瑞生物 | 夏尔巴生物 | 金斯瑞蓬勃生物 | 健元医药 | 星眸生物 | 格兰科医药 | 莱羡科学仪器 | 明度智云 | 玮驰仪器 | 康源久远 | 易慕峰 | 茂行生物 | 济民可信 | 欣协生物 | 泰楚生物 | 泰澧生物 | 谱新生物 | 思鹏生物 | 领诺医药 | 宜明生物 | 爱科瑞思 | 阿思科力 | 博格隆生物 | 百吉生物 | 迈邦生物 | 多宁生物 | 万邦医药 | ASCT | 为度生物 | 比邻星创投 | 赛桥生物 | 吉美瑞生 | 荣泽生物 | 科金生物 | 汉超医药 | 康日百奥 | 汉腾生物 | 力品药业 | 安必生 | 博瑞策生物 | 中盛溯源 | 深研生物 | 东方略 | 赛赋医药 | 克睿基因 | 安润医药 | 镁伽科技 | 科锐迈德 | 和元生物 | 申基生物 |楷拓生物| 森松生命科技 | 凯理斯 | 尚德药缘 | 晟国医药 | 健新原力 | 纽福斯 | 华东医药 | 士泽生物 | 影研医疗科技 | 新格元生物 | 依生生物 | 腾迈医药 | 汉欣医药 | 恒驭生物 | 盛诺基 | 序祯达生物 | 乐纯生物 | 速石科技 | 耀海生物 | 新合生物 | 华龛生物 | 恺佧生物 | 成都凡微析 | 正帆科技 | 大橡科技 | 博雅辑因 | 因美纳 | 博雅控股集团 | 近岸蛋白 | 依科赛生物 | 利穗科技 | 东南科仪 | 倍谙基 | 辉诺医药 | 圣诺制药 | 埃格林医药 | 科镁信 | 爱思益普 | 复星医药 | 齐鲁制药 | 捷思英达丨荣昌生物丨泽璟制药丨奕安济世丨礼新医药丨维立志博丨派格生物丨赛生药业丨呈源生物丨启德医药丨双运生物丨宝船生物丨曙方医药丨澳斯康生物丨普莱医药丨维健医药丨海昶生物丨征祥医药丨智核生物丨望石智慧丨博生吉医药丨南京诺丹丨四星玻璃丨艾米能斯丨霁因生物丨普瑞康生物丨映恩生物丨康哲生物丨霍德生物丨海慈药业丨沃生生物丨睿健医药丨矩阵元丨斯微生物丨则正医药丨预立创投丨东立创新丨博安生物丨伟德杰生物丨星奕昂生物丨耀乘健康科技丨琅钰集团丨康德弘翼 | 原力生命科学丨上海科洲丨特瑞思丨药源丨健艾仕生物丨冠科美博丨微境生物丨天境生物丨合源生物丨泛生子丨创胜集团丨加科思药业丨丹诺医药丨凌科药业丨偶领生物丨凯斯艾生物丨成都圣诺丨松禾资本丨清普生物丨和其瑞丨开拓药业丨科兴制药丨玉森新药丨水木未来丨分享投资丨植德律所丨奥来恩丨乐明药业丨东曜药业丨君圣泰丨海创药业丨天汇资本丨再鼎医药丨济煜医药丨百英生物丨基石药业丨君实生物丨Sirnaomics,Inc.丨亦诺微丨博腾股份丨思路迪诊断丨艾博生物丨普瑞金生物丨未知君生物丨尚健生物丨阿诺医药丨有临医药丨赛业生物丨睿智医药丨博济医药丨晶泰科技丨药明康德丨创志科技丨奥星集团丨苏雅医药丨科贝源丨合全药业丨以岭药业丨科睿唯安丨DRG丨博瑞医药丨丽珠医药丨信立泰药业丨步长制药丨华素制药丨众生药业丨上海医药丨高博医疗集团丨药渡丨君联资本丨集萃药康丨诺思格丨精鼎医药丨百利药业丨Pfizer CentreOne丨默克中国创新中心丨奥来恩丨瑞博生物丨新通药物丨广东中润丨医普科诺丨诺唯赞丨康利华丨国信医药丨昆翎丨博纳西亚丨缔脉丨一品红丨和泽医药丨博志研新丨凯莱英医药丨汉佛莱丨英派药业丨京卫制药丨海思科药业丨宏韧医药丨开心生活科技丨哈三联丨Premier Research丨宣泰医药丨先声药业丨海金格丨普瑞盛医药丨Informa丨科特勒丨谋思医药丨HLT丨莱佛士丨辉瑞丨科林利康丨冠科生物丨科文斯丨卫信康丨龙沙(Lonza)丨美迪西丨阳光诺和丨润东医药丨勃林格殷格翰(中国)丨艾苏莱生物丨领晟医疗丨驯鹿医疗丨燃石医学丨中肽生化丨鸿运华宁丨泰格医药丨易迪希丨希麦迪丨百奥赛图丨迪纳利丨青云瑞晶丨鼎丰生科资本丨中源协和丨维亚生物丨青松医药丨中科谱研丨长风药业丨艾欣达伟丨鼎康生物丨中晟全肽丨海步医药丨勤浩医药丨奥萨医药丨太美医疗科技丨生特瑞丨东富龙丨Cytiva丨优辰实验室丨苏桥生物丨君达合创丨澎立生物丨南京澳健丨南京科默丨东阳光丨亚盛医药丨杰克森实验室丨上海科州丨三优生物丨三迭纪丨泰诺麦博丨Cell Signaling Technology丨PPC佳生丨澳斯康丨先为达丨智享生物丨锐得麦丨宜明昂科丨明济生物丨英百瑞丨六合宁远丨天津天诚丨百拓生物丨星药科技丨亓上生物丨真实生物丨引光医药丨方达医药丨高博医疗集团丨赞荣医药丨国投创新丨药明生物丨康哲药业丨高特佳投资丨普瑞基准丨臻格生物丨微谱医药丨和玉资本 | 倚锋资本
基因疗法临床研究细胞疗法寡核苷酸
2023-11-27
AstraZeneca's rare disease unit has received a recommendation from the National Institute for Health and Care Excellence (NICE) for the use of its enzyme replacement therapy in infants with Wolman disease.
Alexion’s Kanuma (sebelipase alfa), which has been specifically recommended for use in patients who are aged two years or younger when administration begins, will now become the first treatment available on the NHS for the rapidly-progressive rare genetic disease.
Occurring in around one in 350,000 births, Wolman disease causes a build-up of fat in cells in the liver, heart, blood vessels and digestive system.
Symptoms in infants include enlarged liver and spleen, poor weight gain, low muscle tone, jaundice, vomiting, diarrhoea, developmental delay and anaemia.
Until now, standard care for the disease has been palliative and limited to managing symptoms, with patients normally not surviving past the age of one without treatment.
Administered as weekly intravenous infusions which can be given at home alongside a restricted low-fat diet, Kanuma works by replacing an enzyme missing in the body. Some patients may also have a blood and marrow/stem cell transplant.
NHS chief executive, Amanda Pritchard, said: “I am delighted the NHS can now, for the first time, offer a life-changing treatment to families facing this enormously difficult condition.
“Where previously there were no treatments available for infants facing this debilitating disease, this new therapy could save families from facing indescribable grief and allow more children… to grow up, go to school and live normal lives.”
The therapy will now be fast-tracked via NHS England’s Innovative Medicines Fund to enable routine patient access up to five months earlier than would otherwise be the case.
Sean Richardson, vice president and general manager, Alexion, UK, described NICE’s decision as a “milestone moment for infants born with Wolman Disease and their families”.
He continued: “The recommendation is the result of continued constructive collaboration between Alexion, NICE, NHS England, patient groups and the medical community, to ensure babies born with this life-threatening disease have a treatment available to them.”

上市批准
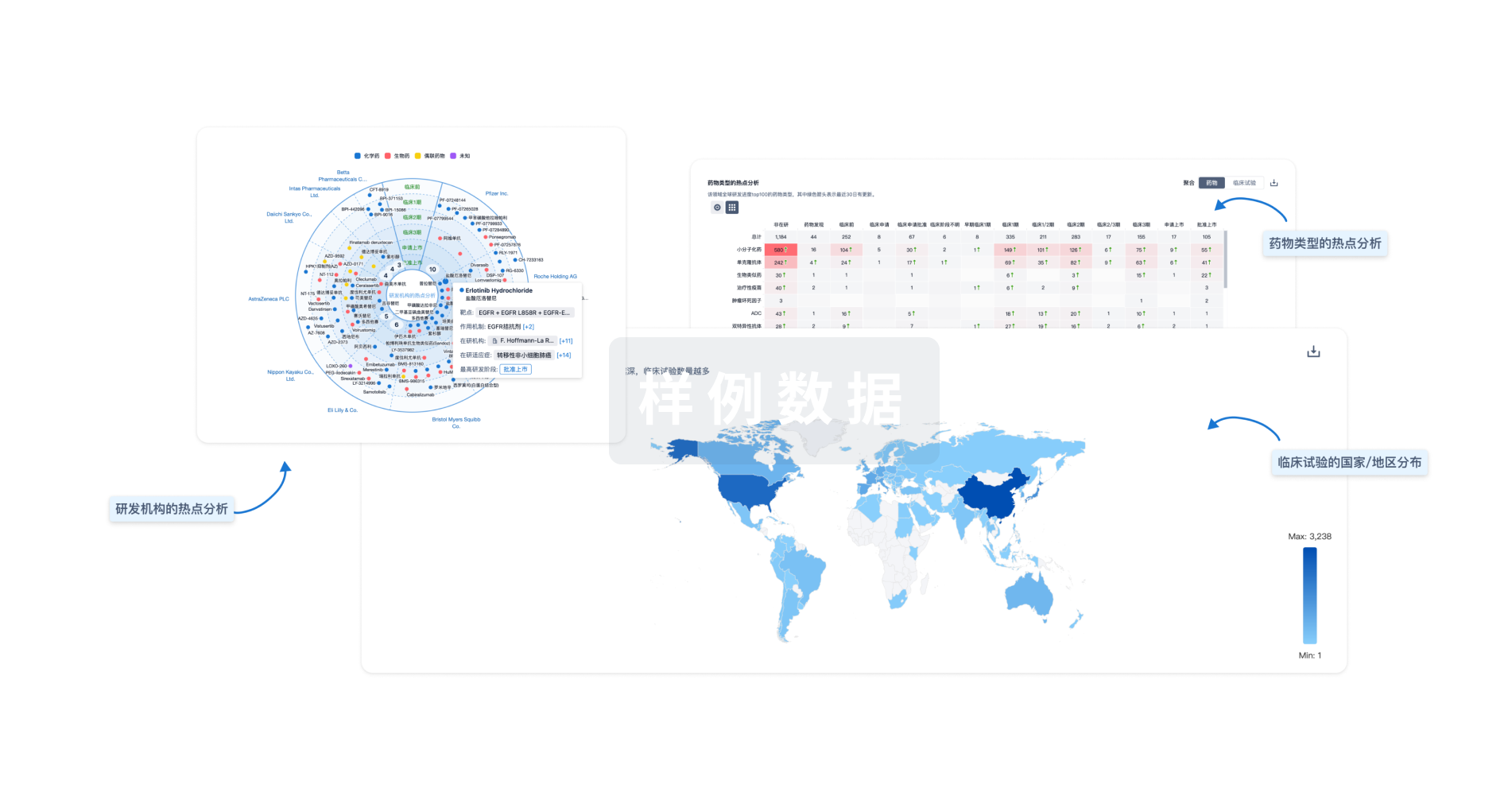
分析
对领域进行一次全面的分析。
登录
或
生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用




