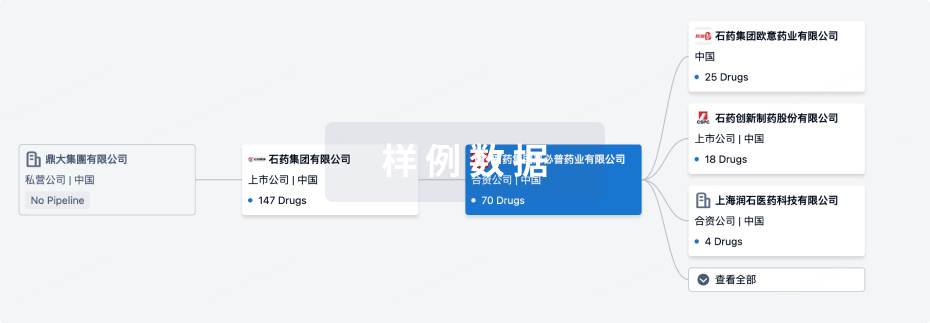
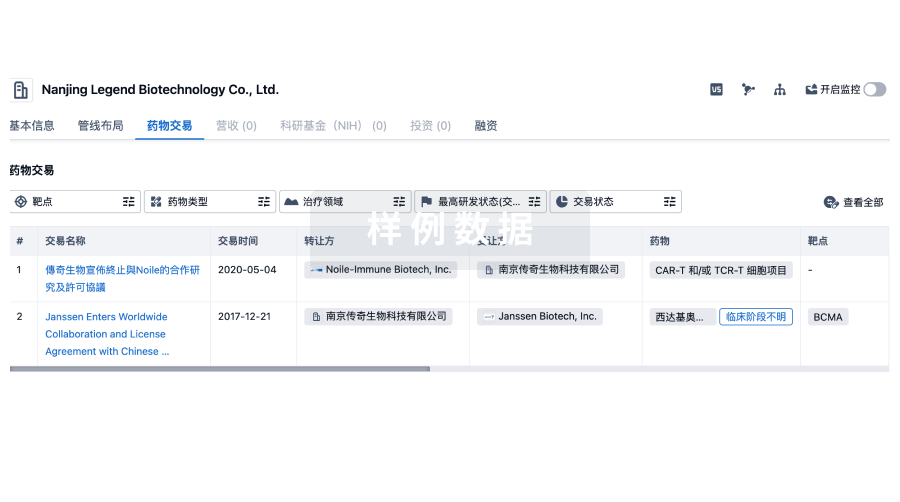
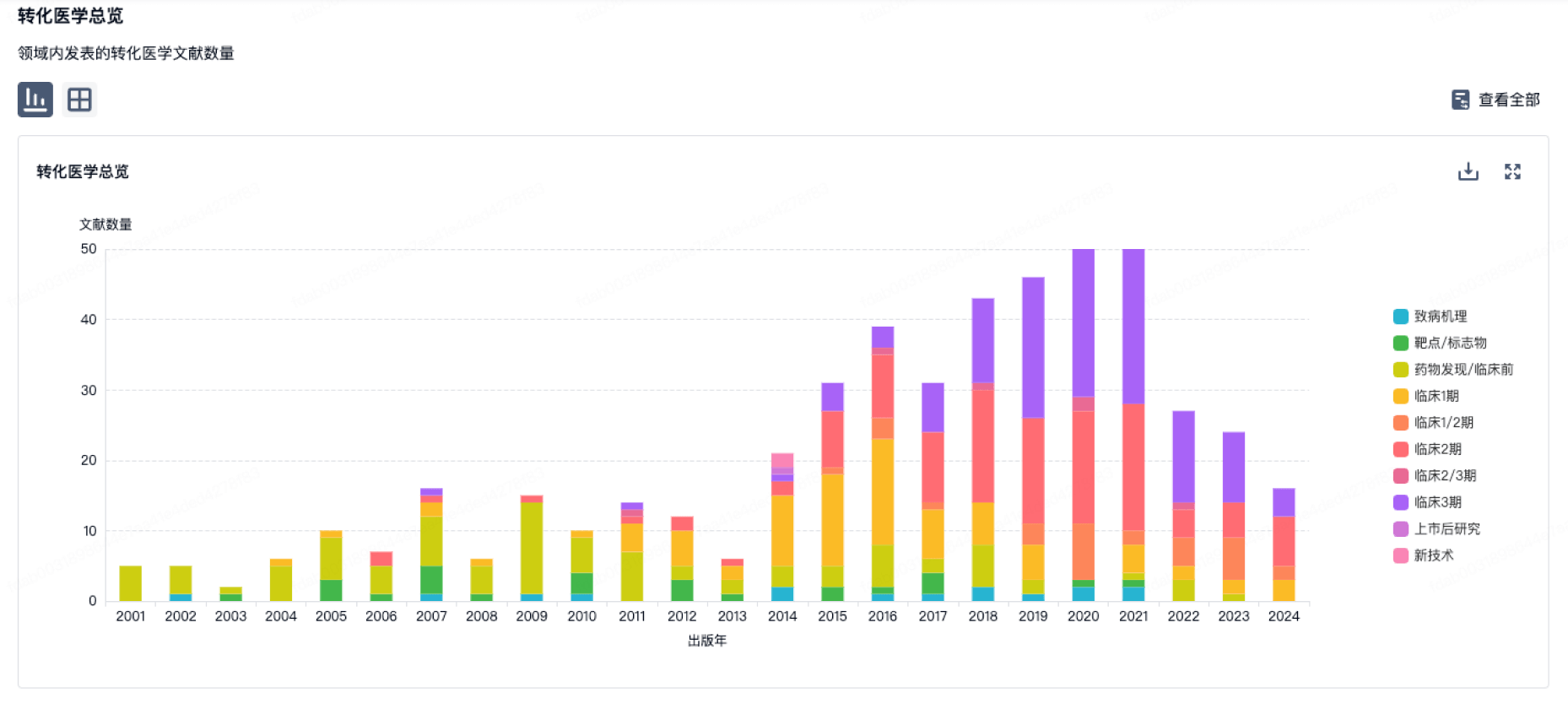
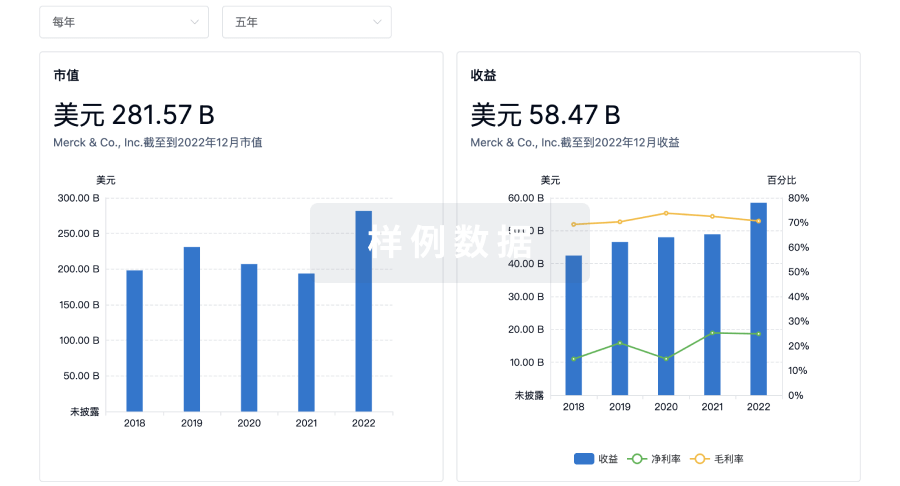
靶点- |
|
|
|
|
|
最高研发阶段批准上市 |
|
首次获批日期2003-12-31 |
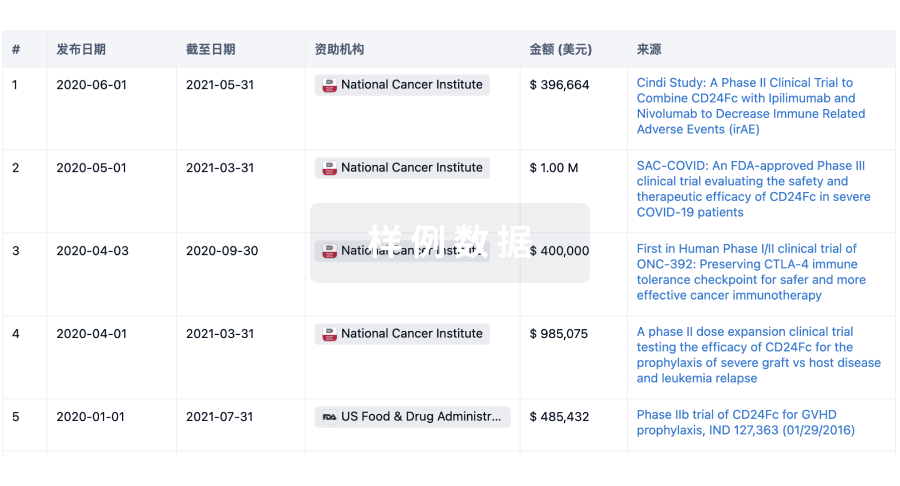
A Phase 1, Randomized, Open-Label, Three-Treatment, Three-Period Crossover Study to Assess Bioequivalence and Safety of TTYP01 Tablets to Radicava® Injection, and Radicava ORS® in Healthy Adult Subjects Under Fasting Conditions
This is a Phase 1, Randomized, Open-Label, Three-Treatment, Three-Period Crossover Study to Assess Bioequivalence and Safety of TTYP01 Tablets to Radicava® Injection, and Radicava ORS® in Healthy Adult Subjects Under Fasting Conditions.The objective is To characterize the bioequivalence、safety and tolerability of TTYP01 tablets and Radicava® injection or Radicava ORS®in healthy adult subjects under fasted conditions.In this study, 30 healthy adult subjects will receive TTYP01, or Radicava, orRadicava ORS in each period according to the randomization sequence.
A Randomized, Double-blind, Placebo-controlled, Multicenter Phase III Clinical Trial to Evaluate the Efficacy and Safety of TTYP01 Tablets in the Treatment of Acute Ischemic Stroke
The study is designed as a multi-center, randomized, double-blind, parallel, placebo-controlled trial to evaluate the efficacy and safety of TTYP01 tablets in the treatment of acute ischemic stroke.
100 项与 Auzone Biological Technology Pty Ltd 相关的临床结果
0 项与 Auzone Biological Technology Pty Ltd 相关的专利(医药)
100 项与 Auzone Biological Technology Pty Ltd 相关的药物交易
100 项与 Auzone Biological Technology Pty Ltd 相关的转化医学