更新于:2024-12-11

Apsen Farmaceutica SA
更新于:2024-12-11
概览
标签
神经系统疾病
化学药
关联
1
项与 Apsen Farmaceutica SA 相关的药物靶点- |
作用机制- |
在研适应症 |
非在研适应症- |
最高研发阶段临床3期 |
首次获批国家/地区- |
首次获批日期- |
14
项与 Apsen Farmaceutica SA 相关的临床试验National, Multicentre, Randomized, Double-blind, Triple-dummy Active-Controled Phase III Clinical Trial to Evaluate the Efficacy and Safety of APSCTC for the Treatment of Postsurgical Pain Due to Third Molar Extraction.
The purpose of this study is to evaluate the efficacy of APSCTC compared to two active drugs in acute pain relief.
开始日期2023-05-13 |
申办/合作机构 |
Multicentre, Open Label, Phase I Clinical Trial to Evaluate the Safety of APSLXR for the Treatment of Vertigo of Vestibular Origin or Meniere's Disease
The purpose of this study is to evaluate the safety of APSLXR in participants with Meniere's disease or other Verigo of vestibular origin. Pharmacokinetics will also be evaluated in a small group.
开始日期2023-01-01 |
申办/合作机构 |
National, Multicentre, Randomized, Double-blind, Double-dummy Phase II Clinical Trial to Evaluate the Efficacy and Safety of S (+) - Ibuprofen for Pain Control in Individuals With Osteoarthritis
The purpose of this study is to evaluate the efficacy of S (+) - ibuprofen compared to an active treatment for pain control in individuals diagnosed with osteoarthritis.
开始日期2022-08-05 |
申办/合作机构 |
100 项与 Apsen Farmaceutica SA 相关的临床结果
登录后查看更多信息
0 项与 Apsen Farmaceutica SA 相关的专利(医药)
登录后查看更多信息
5
项与 Apsen Farmaceutica SA 相关的新闻(医药)2024-12-11
◆ 考察主题:中国药企企业家对话巴西医药企业家——共探两国资源互补合作之旅
◆ 主办单位:药融圈 & 阿萨伊
◆ 考察时间:2025年3月9日-3月15日(7天6夜)
◆ 考察地点:巴西圣保罗
考察背景
巴西,作为金砖国家的重要成员及中国在南美最大的医药贸易伙伴,凭借庞大的人口基数(2.15亿)、持续增长的预期寿命和对健康领域的高度重视(医疗支出占GDP的9.47%),展现出巨大的健康需求和消费潜力,使巴西成为全球医药市场的重要布局点,并吸引了众多中国企业的国际化战略目光。
据IQVIA报告,2023年巴西医药市场规模达到356亿美元,约占全球市场份额的2.2%,是拉美地区最大的医药市场。巴西药品市场以专利药和品牌仿制药为主导,仿制药市场规模占据市场近六成份额。巴西制药行业竞争激烈,拥有349家制药公司,包括众多国际制药公司和本土制药企业。同时,巴西国家卫生监管局(ANVISA)对医药产品和服务的准入标准较高,所有药品和医疗器械的上市必须获得其资质证书,并实施药品专利强制许可和价格调控政策。
中国医药企业通过授权许可、本土化生产和战略并购等方式积极出海巴西,如复宏汉霖和百奥泰采用授权许可模式,甘李药业在巴西与Biomm公司合作进行本土化生产,深耕糖尿病治疗市场,而时代天使则通过战略并购巴西正畸市场领先品牌Aditek 51%的股权。随着中巴关系的显著改善和巴西监管政策的改革以及宏观经济的稳定,中国企业将迎来更加友好的政策环境,包括税收减免、市场准入和通关便利化等政策优惠,同时在消费医疗、慢性疾病治疗和传染病防控等关键领域展现出巨大的市场潜力。
在此背景下,组织一次中国药企巴西考察活动显得尤为重要。通过实地考察巴西知名药企、与当地顶尖医药企业家进行深度对话,以及体验巴西独特的桑巴文化和狂欢节氛围,中国药企可以更加全面地了解巴西医药市场,寻找合作机遇,增进两国企业家之间的友谊与信任。同时,有20年巴西经验的企业带队,将为中国药企提供更加精准、专业的指导和支持,确保考察活动的顺利进行和取得实效。
咨询报名
【备注:公司+姓名+职位】
考察亮点
深度对话
直连巴西顶尖医药企业家,挖掘合作潜力。
企业参访
探访巴西知名药企,学习前沿研发管理。
药监局链接
建立与巴西国家卫生监督局的沟通桥梁。
本土药企合作
链接巴西30家本土药企,拓宽合作网络。
巴西狂欢节
体验2025年巴西狂欢节的桑巴文化盛宴。
工程中心
中国与巴西院士工作站联合的工程中心。
巴西可链接企业介绍
以下排名不分先后,滑动查看更多
Aché Laboratórios
Aché Laboratórios成立于1966年,巴西五大基地,产品超157类,涉及30医域,创新高质量,国际影响力大。
Apsen Farmacêutica
Apsen Farmacêutica成立于1969年,巴西本土制药公司,产品多样,涵盖多治疗领域,凭借专业知识和质量承诺领先市场。
EMS Pharma Farmaceutica
EMS制药1964年创于巴西,产品覆盖广泛,创新领先,销往40余国,近期拟合并Hypera Pharma,是巴西全球制药重要参与者。
Eurofarma
Eurofarma1972年创于巴西,产品超2000种,服务42医专,拉美24国业务,2023年净销91亿雷亚尔,拉美领先制药公司。
Hypera Pharma
Hypera Pharma成立于2001年,巴西制药领导者,产品涵盖OTC、处方药等,拥有知名品牌,现代化研发中心,强大生产能力。
Laboratório TeutoBrasileiro
Teuto制药1947年成立,巴西领先,产品涵盖处方药等540余种,严格质控,出口多国,巴西国际信赖制药公司。
LIBBS FARMACÊUTICALTDA
Libbs成立于1958年,巴西医药解决方案生产商,2000+员工,100+品牌,生产生物仿制药,对复杂疾病治疗至关重要。
Medley S/A
Medley制药1996年成立,巴西仿制药领导者,2009年被赛诺菲收购,保持品牌独立,致力于研发创新,提供多样化治疗选择。
Laboratório NeoQuímica Comércioe Indústria
Neo Química制药1959年成立,巴西仿制药领导者,2009年被Hypera收购,保持品牌独立,致力于研发创新,提供多样化治疗。
Novo NordiskFarmacêuticado Brasil Ltda.
诺和诺德1923年成立,专注慢性病治疗,2024年投资巴西工厂升级,提供多样化治疗方案。
Sanofi MedleyFarmacêutica Ltda.
Medley制药1996年成立,2009年被赛诺菲收购,专注高质量仿制药,成巴西制药行业重要参与者。
Autocam Medical
Autocam Medical制造高精度医疗部件,如骨科植入物、器械,全球运营,巴西工厂符合ANVISA标准。
Biomarin BrasilFarmacêutica
BioMarin自1997年专注罕见遗传病药物研发,2007年进入巴西,提供多种创新疗法,2024年基因疗法RoctavianR获批。
Biomm S/A
Biomm S/A2001年成立于巴西,专注慢性病创新药物,涵盖胰岛素类似物,致力于生物技术开发与商业化。
Bionovis SA
Bionovis S.A.2012年由四家巴西药企创立,专注研发生产高复杂度生物药,旨在减少进口依赖,增强生物制药自主性。
Chiesi Farmacêutica
凯西制药1935年成立,专注呼吸健康、罕见病和专科治疗,2023年收购Amryt Pharma,全球31国设分公司,积极履行社会责任。
ELS Solutions
ELS Solutions是制药健康医疗技术咨询公司,设欧美拉美分支,提供研发、市场、法规等全面服务。
Fundação Osvaldo Cruz
Fiocruz成立于1900年,是巴西生物医学研究及疫苗生产领军机构,疫情期间为COVID-19防控做出重大贡献。
Fresenius Kabi Brasil
Fresenius Kabi Brasil专注医疗保健,提供输液、输血、临床营养及生物制药,2022年与Fiocruz等合作生产阿达木单抗生物类似药。
Laboratório Fleury Brasil
Fleury Medicina e Saúde成立于1926年,是巴西领先的医学诊断公司,提供实验室、影像、疫苗、体检、基因组学和生育治疗服务。
Hospital
Sírio-Libanês|BelaVista
Hospital Sírio-Libanês成立于1921年,是圣保罗知名医疗机构,提供多学科医疗服务,以卓越医疗和人性化护理闻名。
InterfarmaImportação e Exportaçãode Medicamentos
Interfarma成立于1987年,是巴西圣保罗的药品进出口公司,协助进口未注册或短缺药品,并出口合规药品。
Novozymes LatinAmerica Ltda
Novozymes Latin America Ltda.是诺维信巴西子公司,1975年成立,为多个行业提供酶和微生物解决方案,推动行业发展。
RD Saúde-CD Goiás
RD Saúde是巴西药房连锁集团,2011年由Raia和Drogasil合并,拥有14个配送中心,戈亚斯州为其一,加强中西部物流。
SinovaSaúde-Departamentode Inovação UFSC
SINOVA是UFSC创新部门,2024年推出PrevCancer项目,负责技术转移、知识产权管理,支持创业,促进产学研合作。
Tanner Pharma Group
Tanner Pharma Group成立于2002年,提供全球医疗保健解决方案,包括药物获取计划、临床试验采购和商业化服务,助力患者获取关键药物。
UnitherPharmaceuticals Brasil
Unither Pharmaceuticals是国际制药公司,在巴西有两家工厂,专注生产医疗解决方案,加强拉美市场地位。
Veeva Systems
Veeva Systems成立于2007年,为制药和生命科学行业提供SaaS云解决方案,包括商务、研发和医学云,全球超1300家客户。
West PharmaceuticalServices Brasil
西氏制药服务巴西公司1974年成立,生产高质量医疗组件,提供注射药物包装和给药系统,支持当地慈善。
咨询报名
【备注:公司+姓名+职位】
日程安排
DAY 1 (3月9日,星期日)
抵达巴西圣保罗,接机并入住酒店。
晚上举行欢迎晚宴,增进彼此了解。
DAY 2 (3月10日,星期一)
● 上午 探索本地药企:巴西制药领袖的创新与广泛产品线
● 下午 探索巴西制药巨头的创新之路:从研发中心到全球市场拓展及潜在合并机遇
● 晚上 品味巴西风情:揭秘圣保罗地道烤肉美食之旅
DAY 3 (3月11日,星期二)
● 上午 药监局链接:探讨巴西药监局对中国医药行业进入巴西市场的政策理解与未来规划
● 下午 实地走访:探索巴西本土制药实验室的辉煌历程与全球影响力
● 晚上 巴西海岸风味:探寻海鲜炖菜的独特魅力
DAY 4 (3月12日,星期三)
● 上午 深入探访本土企业:巴西制药领导者的创新之路与生产实力
● 下午 实地探访巴西领先制药企业:探索制药创新与全球影响力
● 晚上 巴西传统美食探秘:品味豆子大乱炖的葡萄牙风情
DAY 5 (3月13日,星期四)
● 上午 深入探访本土制药实验室:学习运营精髓,探索合作机遇与评估投资潜力
● 下午 实地走访本土药企:了解巴西仿制药市场领导者的现代化生产与研发创新
● 晚上 巴西美食探秘:从巧克力球到多元风味的美食之旅
DAY 6 (3月14日,星期五)
● 上午 热情桑巴,学习之旅:分享考察中的所见所闻所感
● 下午 圣保罗探秘:解锁巴西城市魅力之旅
● 晚上 舞动桑巴灵魂,探秘圣保罗狂欢:一场穿越巴西热情的梦幻之旅!
DAY 7 (3月15日,星期六)
离开巴西圣保罗,送机,结束考察活动。
注意事项:实际日程请以最终确认为准,主办方保留对行程安排进行合理调整的权利。
咨询报名
【备注:公司+姓名+职位】
报名详情
活动时间:
2025年3月9日-3月15日(7天6夜)
活动地点:
巴西圣保罗
参加人员:
◎ 有意突破国内困局,开拓巴西市场的医药企业;
◎ 原料药、制剂、制药设备生产商、中医诊疗机构等;
◎ 探索医药巴西本土化生产的决策者;
◎ 有意在巴西当地定制产品,销售或者外销的投资者:
◎ 对巴西设立办事处/公司有兴趣的投资者等
◎ 有意链接中巴院工程中心的企业家或投资者
报名方式
咨询报名
【备注:公司+姓名+职位】
这是一次难得的跨国交流机会,让我们携手共进,探索中国与巴西医药产业合作的新篇章!期待您的加入,共同开启这段充满机遇与挑战的考察之旅!
往届出海考察活动
越南行“完美收官”| 2024药融圈企业家医药出海战略
老挝卫生部部长本丰·普玛莱接见中国医药企业考察团,开启中国药企出海新篇章!
注:本文部分信息源于网络,版权归原作者,侵权请联系删除。
参考来源:https://mp.weixin.qq.com/s/2w9q-7FJS0Zhf9zM0Zobtw
并购
2024-12-11
·药融圈
◆ 考察主题:中国药企企业家对话巴西医药企业家——共探两国资源互补合作之旅
◆ 主办单位:药融圈 & 阿萨伊
◆ 考察时间:2025年3月9日-3月15日(7天6夜)
◆ 考察地点:巴西圣保罗
考察背景
巴西,作为金砖国家的重要成员及中国在南美最大的医药贸易伙伴,凭借庞大的人口基数(2.15亿)、持续增长的预期寿命和对健康领域的高度重视(医疗支出占GDP的9.47%),展现出巨大的健康需求和消费潜力,使巴西成为全球医药市场的重要布局点,并吸引了众多中国企业的国际化战略目光。
据IQVIA报告,2023年巴西医药市场规模达到356亿美元,约占全球市场份额的2.2%,是拉美地区最大的医药市场。巴西药品市场以专利药和品牌仿制药为主导,仿制药市场规模占据市场近六成份额。巴西制药行业竞争激烈,拥有349家制药公司,包括众多国际制药公司和本土制药企业。同时,巴西国家卫生监管局(ANVISA)对医药产品和服务的准入标准较高,所有药品和医疗器械的上市必须获得其资质证书,并实施药品专利强制许可和价格调控政策。
中国医药企业通过授权许可、本土化生产和战略并购等方式积极出海巴西,如复宏汉霖和百奥泰采用授权许可模式,甘李药业在巴西与Biomm公司合作进行本土化生产,深耕糖尿病治疗市场,而时代天使则通过战略并购巴西正畸市场领先品牌Aditek 51%的股权。随着中巴关系的显著改善和巴西监管政策的改革以及宏观经济的稳定,中国企业将迎来更加友好的政策环境,包括税收减免、市场准入和通关便利化等政策优惠,同时在消费医疗、慢性疾病治疗和传染病防控等关键领域展现出巨大的市场潜力。
在此背景下,组织一次中国药企巴西考察活动显得尤为重要。通过实地考察巴西知名药企、与当地顶尖医药企业家进行深度对话,以及体验巴西独特的桑巴文化和狂欢节氛围,中国药企可以更加全面地了解巴西医药市场,寻找合作机遇,增进两国企业家之间的友谊与信任。同时,有20年巴西经验的企业带队,将为中国药企提供更加精准、专业的指导和支持,确保考察活动的顺利进行和取得实效。
考察亮点
深度对话
直连巴西顶尖医药企业家,挖掘合作潜力。
企业参访
探访巴西知名药企,学习前沿研发管理。
药监局链接
建立与巴西国家卫生监督局的沟通桥梁。
本土药企合作
链接巴西30家本土药企,拓宽合作网络。
巴西狂欢节
体验2025年巴西狂欢节的桑巴文化盛宴。
工程中心
中国与巴西院士工作站联合的工程中心。
巴西可链接企业介绍
以下排名不分先后,滑动查看更多
Aché Laboratórios
Aché Laboratórios成立于1966年,巴西五大基地,产品超157类,涉及30医域,创新高质量,国际影响力大。
Apsen Farmacêutica
Apsen Farmacêutica成立于1969年,巴西本土制药公司,产品多样,涵盖多治疗领域,凭借专业知识和质量承诺领先市场。
EMS Pharma Farmaceutica
EMS制药1964年创于巴西,产品覆盖广泛,创新领先,销往40余国,近期拟合并Hypera Pharma,是巴西全球制药重要参与者。
Eurofarma
Eurofarma1972年创于巴西,产品超2000种,服务42医专,拉美24国业务,2023年净销91亿雷亚尔,拉美领先制药公司。
Hypera Pharma
Hypera Pharma成立于2001年,巴西制药领导者,产品涵盖OTC、处方药等,拥有知名品牌,现代化研发中心,强大生产能力。
Laboratório TeutoBrasileiro
Teuto制药1947年成立,巴西领先,产品涵盖处方药等540余种,严格质控,出口多国,巴西国际信赖制药公司。
LIBBS FARMACÊUTICALTDA
Libbs成立于1958年,巴西医药解决方案生产商,2000+员工,100+品牌,生产生物仿制药,对复杂疾病治疗至关重要。
Medley S/A
Medley制药1996年成立,巴西仿制药领导者,2009年被赛诺菲收购,保持品牌独立,致力于研发创新,提供多样化治疗选择。
Laboratório NeoQuímica Comércioe Indústria
Neo Química制药1959年成立,巴西仿制药领导者,2009年被Hypera收购,保持品牌独立,致力于研发创新,提供多样化治疗。
Novo NordiskFarmacêuticado Brasil Ltda.
诺和诺德1923年成立,专注慢性病治疗,2024年投资巴西工厂升级,提供多样化治疗方案。
Sanofi MedleyFarmacêutica Ltda.
Medley制药1996年成立,2009年被赛诺菲收购,专注高质量仿制药,成巴西制药行业重要参与者。
Autocam Medical
Autocam Medical制造高精度医疗部件,如骨科植入物、器械,全球运营,巴西工厂符合ANVISA标准。
Biomarin BrasilFarmacêutica
BioMarin自1997年专注罕见遗传病药物研发,2007年进入巴西,提供多种创新疗法,2024年基因疗法RoctavianR获批。
Biomm S/A
Biomm S/A2001年成立于巴西,专注慢性病创新药物,涵盖胰岛素类似物,致力于生物技术开发与商业化。
Bionovis SA
Bionovis S.A.2012年由四家巴西药企创立,专注研发生产高复杂度生物药,旨在减少进口依赖,增强生物制药自主性。
Chiesi Farmacêutica
凯西制药1935年成立,专注呼吸健康、罕见病和专科治疗,2023年收购Amryt Pharma,全球31国设分公司,积极履行社会责任。
ELS Solutions
ELS Solutions是制药健康医疗技术咨询公司,设欧美拉美分支,提供研发、市场、法规等全面服务。
Fundação Osvaldo Cruz
Fiocruz成立于1900年,是巴西生物医学研究及疫苗生产领军机构,疫情期间为COVID-19防控做出重大贡献。
Fresenius Kabi Brasil
Fresenius Kabi Brasil专注医疗保健,提供输液、输血、临床营养及生物制药,2022年与Fiocruz等合作生产阿达木单抗生物类似药。
Laboratório Fleury Brasil
Fleury Medicina e Saúde成立于1926年,是巴西领先的医学诊断公司,提供实验室、影像、疫苗、体检、基因组学和生育治疗服务。
Hospital
Sírio-Libanês|BelaVista
Hospital Sírio-Libanês成立于1921年,是圣保罗知名医疗机构,提供多学科医疗服务,以卓越医疗和人性化护理闻名。
InterfarmaImportação e Exportaçãode Medicamentos
Interfarma成立于1987年,是巴西圣保罗的药品进出口公司,协助进口未注册或短缺药品,并出口合规药品。
Novozymes LatinAmerica Ltda
Novozymes Latin America Ltda.是诺维信巴西子公司,1975年成立,为多个行业提供酶和微生物解决方案,推动行业发展。
RD Saúde-CD Goiás
RD Saúde是巴西药房连锁集团,2011年由Raia和Drogasil合并,拥有14个配送中心,戈亚斯州为其一,加强中西部物流。
SinovaSaúde-Departamentode Inovação UFSC
SINOVA是UFSC创新部门,2024年推出PrevCancer项目,负责技术转移、知识产权管理,支持创业,促进产学研合作。
Tanner Pharma Group
Tanner Pharma Group成立于2002年,提供全球医疗保健解决方案,包括药物获取计划、临床试验采购和商业化服务,助力患者获取关键药物。
UnitherPharmaceuticals Brasil
Unither Pharmaceuticals是国际制药公司,在巴西有两家工厂,专注生产医疗解决方案,加强拉美市场地位。
Veeva Systems
Veeva Systems成立于2007年,为制药和生命科学行业提供SaaS云解决方案,包括商务、研发和医学云,全球超1300家客户。
West PharmaceuticalServices Brasil
西氏制药服务巴西公司1974年成立,生产高质量医疗组件,提供注射药物包装和给药系统,支持当地慈善。
日程安排
DAY 1 (3月9日,星期日)
抵达巴西圣保罗,接机并入住酒店。
晚上举行欢迎晚宴,增进彼此了解。
DAY 2 (3月10日,星期一)
● 上午 探索本地药企:巴西制药领袖的创新与广泛产品线
● 下午 探索巴西制药巨头的创新之路:从研发中心到全球市场拓展及潜在合并机遇
● 晚上 品味巴西风情:揭秘圣保罗地道烤肉美食之旅
DAY 3 (3月11日,星期二)
● 上午 药监局链接:探讨巴西药监局对中国医药行业进入巴西市场的政策理解与未来规划
● 下午 实地走访:探索巴西本土制药实验室的辉煌历程与全球影响力
● 晚上 巴西海岸风味:探寻海鲜炖菜的独特魅力
DAY 4 (3月12日,星期三)
● 上午 深入探访本土企业:巴西制药领导者的创新之路与生产实力
● 下午 实地探访巴西领先制药企业:探索制药创新与全球影响力
● 晚上 巴西传统美食探秘:品味豆子大乱炖的葡萄牙风情
DAY 5 (3月13日,星期四)
● 上午 深入探访本土制药实验室:学习运营精髓,探索合作机遇与评估投资潜力
● 下午 实地走访本土药企:了解巴西仿制药市场领导者的现代化生产与研发创新
● 晚上 巴西美食探秘:从巧克力球到多元风味的美食之旅
DAY 6 (3月14日,星期五)
● 上午 热情桑巴,学习之旅:分享考察中的所见所闻所感
● 下午 圣保罗探秘:解锁巴西城市魅力之旅
● 晚上 舞动桑巴灵魂,探秘圣保罗狂欢:一场穿越巴西热情的梦幻之旅!
DAY 7 (3月15日,星期六)
离开巴西圣保罗,送机,结束考察活动。
注意事项:实际日程请以最终确认为准,主办方保留对行程安排进行合理调整的权利。
报名详情
活动时间:
2025年3月9日-3月15日(7天6夜)
活动地点:
巴西圣保罗
参加人员:
◎ 有意突破国内困局,开拓巴西市场的医药企业;
◎ 原料药、制剂、制药设备生产商、中医诊疗机构等;
◎ 探索医药巴西本土化生产的决策者;
◎ 有意在巴西当地定制产品,销售或者外销的投资者:
◎ 对巴西设立办事处/公司有兴趣的投资者等
◎ 有意链接中巴院工程中心的企业家或投资者
报名方式
长按二维码,填写报名表
更多问题,请联系咨询报名
【备注:公司+姓名+职位】
这是一次难得的跨国交流机会,让我们携手共进,探索中国与巴西医药产业合作的新篇章!期待您的加入,共同开启这段充满机遇与挑战的考察之旅!
往届出海考察活动
越南行“完美收官”| 2024药融圈企业家医药出海战略
老挝卫生部部长本丰·普玛莱接见中国医药企业考察团,开启中国药企出海新篇章!
注:本文部分信息源于网络,版权归原作者,侵权请联系删除。
参考来源:https://mp.weixin.qq.com/s/2w9q-7FJS0Zhf9zM0Zobtw
并购抗体药物偶联物
2023-11-15
DUBLIN, Nov. 14, 2023 /PRNewswire/ -- The "Drug Delivery Collaboration and Licensing Deals 2016-2023" report has been added to
ResearchAndMarkets.com's offering.
Drug Delivery Collaboration and Licensing Deals provides a comprehensive understanding and unprecedented access to the drug delivery deals entered into by the worlds leading biopharma companies.
This report contains a comprehensive listing of 1551 drug delivery deals announced since 2016 including financial terms where available including links to online deal records of actual drug delivery partnering deals as disclosed by the deal parties.
Fully revised and updated, the report provides details of drug delivery deals from 2016 to 2023. The report provides a detailed understanding and analysis of how and why companies enter drug delivery deals. These deals tend to be multicomponent, starting with collaborative R&D, and commercialization of outcomes.
The report includes collaboration, development, research and licensing deals. In addition, where available, records include contract documents as submitted to the Securities Exchange Commission by companies and their partners.
The initial chapters of this report provide an orientation of drug delivery dealmaking. The report also includes numerous table and figures that illustrate the trends and activities in drug delivery deal making since 2016.
In addition, a comprehensive deal directory is provided organized by company A-Z, deal type and therapeutic target. Each deal title links via Weblink to an online version of the deal record and where available, the contract document, providing easy access to each contract document on demand.
Key benefits
Understand deal trends since 2016
Browse drug delivery collaboration and licensing deals
Benchmark analysis - identify market value of transactions
Financials terms - upfront, milestone, royalties
Directory of deals by company A-Z, deal type and therapy area
Leading deals by value
Most active dealmakers
Identify assets and deal terms for each transaction
Access contract documents - insights into deal structures
Due diligence - assess suitability of your proposed deal terms for partner companies
Save hundreds of hours of research time
Analyzing contract agreements allows due diligence of:
What are the precise rights granted or optioned?
What is actually granted by the agreement to the partner company?
What exclusivity is granted?
What is the payment structure for the deal?
How are sales and payments audited?
What is the deal term?
How are the key terms of the agreement defined?
How are IPRs handled and owned?
Who is responsible for commercialization?
Who is responsible for development, supply, and manufacture?
How is confidentiality and publication managed?
How are disputes to be resolved?
Under what conditions can the deal be terminated?
What happens when there is a change of ownership?
What sublicensing and subcontracting provisions have been agreed?
Which boilerplate clauses does the company insist upon?
Which boilerplate clauses appear to differ from partner to partner or deal type to deal type?
Which jurisdiction does the company insist upon for agreement law?
Key Topics Covered:
Executive Summary
Chapter 1 - Introduction
Chapter 2 - Trends in drug delivery dealmaking
2.1. Introduction
2.2. Drug delivery deals over the years
2.3. Most active drug delivery dealmakers
2.4. Drug delivery deals by deal type
2.5. Drug delivery deals by therapy area
2.6. Drug delivery deals by industry sector
2.7. Deal terms for drug delivery deals
2.7.1 Drug delivery deals headline values
2.7.2 Drug delivery deal upfront payments
2.7.3 Drug delivery deal milestone payments
2.7.4 Drug delivery royalty rates
Chapter 3 - Leading drug delivery deals
3.1. Introduction
3.2. Top drug delivery deals by value
Chapter 4 - Most active drug delivery dealmakers
4.1. Introduction
4.2. Most active drug delivery dealmakers
4.3. Most active drug delivery deals company profiles
Chapter 5 - Drug delivery contracts dealmaking directory
5.1. Introduction
5.2. Drug delivery contracts dealmaking directory
Chapter 6 - Drug delivery dealmaking by technology type
Deal directory
Deal directory - Drug delivery deals by company A-Z
Deal directory - Drug delivery deals by deal type
Deal directory - Drug delivery deals by therapy area
Companies Mentioned
Delta 9 Cannabis
Korea Advanced Institute of Science and Technology
STADA Arzneimittel
TFB & Associates
Imbed Bio
Sesen Bio
Motif Bio
Avior Bio
Tetra Bio-Pharma
Helix BioPharma
Carna BioSciences
AMPEL BioSolutions
Leads Biolabs
Orbit Biomedical
Proxy Biomedical
Dance Biopharm
NEMUS Bioscience
Artes Biotechnology
Tamir Biotechnology
Juyou Biotechnology
Small Business Innovation Research
Auxly Cannabis Group
North Carolina State University
Magle Chemoswed
LatAm Clinical Trials
Eve & Co
Aceto Corporation
Green Cross LabCell
Nitto Denko
Kwang Dong Pharmaceutical
Rapid Dose Therapeutics
Fidia Farmaceutici
Apsen Farmacutica
Grupo Ferrer
Altus Formulation
Aspen Global
Eitan Group
Clear Guide Medical
Evero Health
World Health Organization
Bayer Healthcare
For more information about this report visit
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
Media Contact:
Research and Markets
Laura Wood, Senior Manager
[email protected]
For E.S.T Office Hours Call +1-917-300-0470
For U.S./CAN Toll Free Call +1-800-526-8630
For GMT Office Hours Call +353-1-416-8900
U.S. Fax: 646-607-1907
Fax (outside U.S.): +353-1-481-1716
SOURCE Research and Markets
100 项与 Apsen Farmaceutica SA 相关的药物交易
登录后查看更多信息
100 项与 Apsen Farmaceutica SA 相关的转化医学
登录后查看更多信息
组织架构
使用我们的机构树数据加速您的研究。
登录
或
管线布局
2024年12月25日管线快照
管线布局中药物为当前组织机构及其子机构作为药物机构进行统计,早期临床1期并入临床1期,临床1/2期并入临床2期,临床2/3期并入临床3期
临床3期
1
2
其他
登录后查看更多信息
药物交易
使用我们的药物交易数据加速您的研究。
登录
或
转化医学
使用我们的转化医学数据加速您的研究。
登录
或
营收
使用 Synapse 探索超过 36 万个组织的财务状况。
登录
或
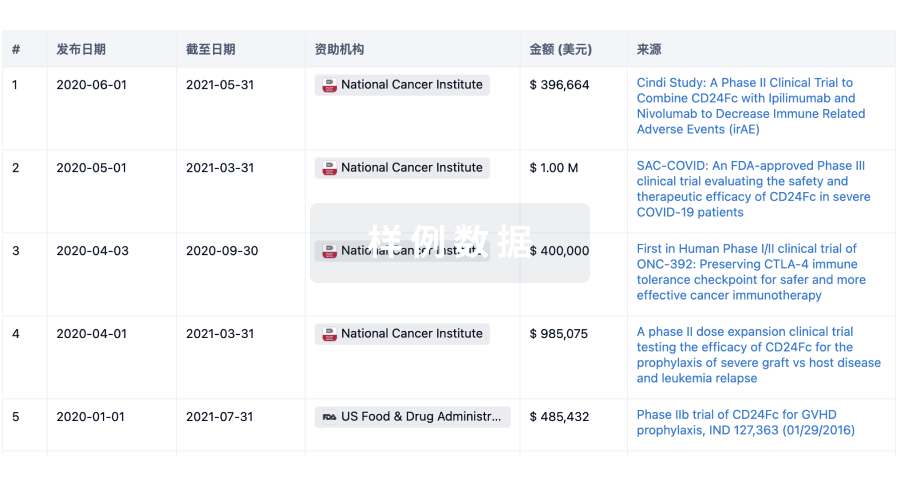
科研基金(NIH)
访问超过 200 万项资助和基金信息,以提升您的研究之旅。
登录
或
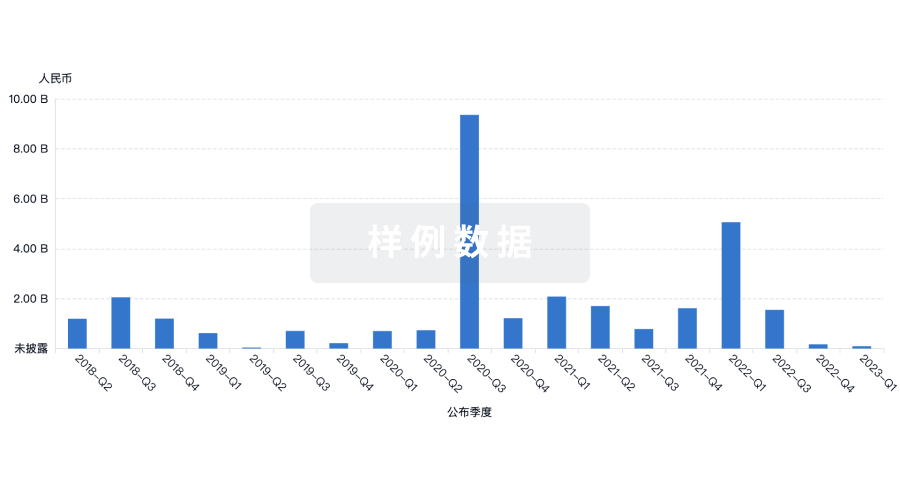
投资
深入了解从初创企业到成熟企业的最新公司投资动态。
登录
或
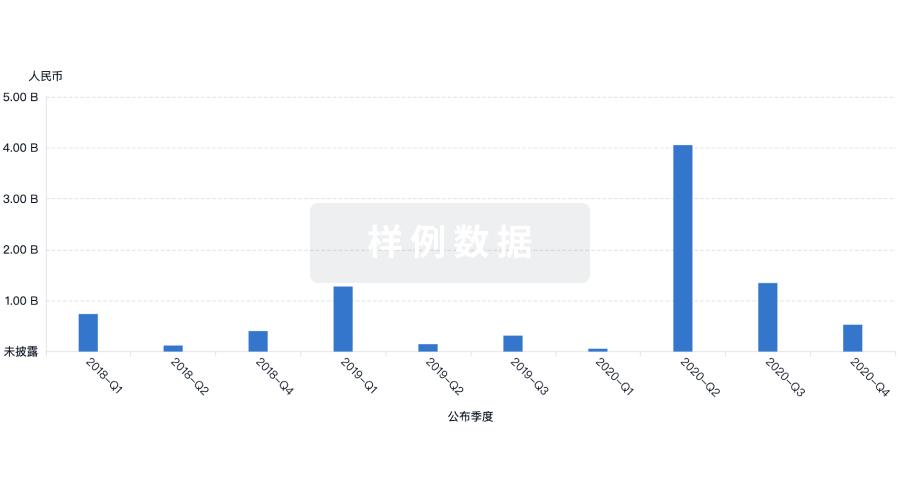
融资
发掘融资趋势以验证和推进您的投资机会。
登录
或
标准版
¥16800
元/账号/年
新药情报库 | 省钱又好用!
立即使用
来和芽仔聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用