更新于:2024-12-18

In3Bio Research Ltd.
更新于:2024-12-18
概览
关联
3
项与 In3Bio Research Ltd. 相关的临床试验A Multicentre, Open-Label, Exploratory Phase Ib Clinical Study to Assess Safety and Efficacy of an EGFR Tyrosine Kinase Inhibitor in Combination With EGF Pathway Targeting Immunisation (EGF-PTI) in Treatment-Naïve Patients With EGFR Mutant NSCLC. The EPICAL Study
This is a multicentre, open-label, uncontrolled, Phase Ib clinical study. Patients who give informed consent will be screened for the study, including genotyping of the tumour and baseline characteristics. Eligible patients will receive a single pre-treatment of low dose of intravenous cyclophosphamide 200 mg/m2 (Day -3). Patients will commence daily oral therapy with the EGFR TKI afatinib as soon as possible, preferably on the same day as low dose cyclophosphamide. Afatinib will be prescribed according to the Summary of Product Characteristics (SmPC) of the product, and will continue in nominal 21-day cycles for as long as clinically indicated. The first day of dosing with EGF-PTI will be designated Day 1.
Immunisation with EGF-PTI will commence 3 days after low dose cyclophosphamide and commencement of EGFR TKI, and will be repeated on Day 14, Day 28, Day 43, and Day 92. After the 5 th vaccination, patients will be followed up every 6 weeks for basic safety data and every 3 months for complete efficacy data, safety data, and maintenance (reduced) doses of EGF-PTI. Patients will continue in the study until disease progression, death, safety concerns (in the opinion of the investigator), non-compliance with the protocol, the patient withdraws from the study, 1 year after randomisation of the last patient, or the study is stopped the sponsor, whichever occurs sooner
Immunisation with EGF-PTI will commence 3 days after low dose cyclophosphamide and commencement of EGFR TKI, and will be repeated on Day 14, Day 28, Day 43, and Day 92. After the 5 th vaccination, patients will be followed up every 6 weeks for basic safety data and every 3 months for complete efficacy data, safety data, and maintenance (reduced) doses of EGF-PTI. Patients will continue in the study until disease progression, death, safety concerns (in the opinion of the investigator), non-compliance with the protocol, the patient withdraws from the study, 1 year after randomisation of the last patient, or the study is stopped the sponsor, whichever occurs sooner
开始日期2018-07-06 |
申办/合作机构 |
Phase 3 Open-label, Multicentre, Randomised Trial to Establish Safety & Efficacy of an EGF Cancer Vaccine in Inoperable, Stage IV Biomarker Positive,Wild Type EGF-R NSCLC Patients Eligible to Receive Standard Treatment and Supportive Care
The vaccine contains humanized recombinant antigen (EGF - Epithelial Growth Factor) and an adjuvant. The antibodies induced by vaccination will react with circulating EGF leading to removal of EGF from the circulation. As a result, binding to its target EGF-Receptor is prevented. Blocking of EGF-Receptor is preventing activation and stimulation of proliferation of tumour cell. A Phase 3 clinical trial on the EGF vaccine is ongoing in Cuba. The result from previous studies demonstrated positive correlation between extended survival and immune response against the vaccination in the late-stage NSCLC patients' age below 60 with improved quality of life. The purpose of this international Phase 3 trial is to determine whether the recombinant human EGF cancer vaccine is safe, immunogenic and effective in the treatment of stage IV NSCLC patients who are positive in the selective EGF biomarker and wild type EGF-Receptor compared to standard treatment and supportive care.
开始日期2015-05-01 |
申办/合作机构 |
Phase III, Open-label, Multicentre, Randomised Trial to Establish Safety and Efficacy of an EGF Cancer Vaccine in Inoperable, Late Stage (IIIb/IV) NSCLC Patients Eligible to Receive Standard Treatment and Supportive Care.
The vaccine contains humanized recombinant antigen (Epithelial Growth Factor) and an adjuvant. The antibodies induced by vaccination will react with circulating EGF leading to removal of EGF from the circulation. As a result, binding to its target EGF-Receptor is prevented. Blocking of EGF-Receptor is preventing activation and stimulation of proliferation of tumour cell. A Phase III clinical trial on the EGF vaccine is ongoing in Cuba. The result from previous studies demonstrated positive correlation between extended survival and immune response against the vaccination in the late-stage NSCLC patients' age below 60 with improved quality of life. The purpose of this international Phase III trial is to determine whether the recombinant human EGF cancer vaccine is safe, immunogenic and effective in the treatment of stage IIIB/IV NSCLC patients compared to standard treatment and supportive care.
开始日期2011-11-01 |
申办/合作机构 |
100 项与 In3Bio Research Ltd. 相关的临床结果
登录后查看更多信息
0 项与 In3Bio Research Ltd. 相关的专利(医药)
登录后查看更多信息
100 项与 In3Bio Research Ltd. 相关的药物交易
登录后查看更多信息
100 项与 In3Bio Research Ltd. 相关的转化医学
登录后查看更多信息
组织架构
使用我们的机构树数据加速您的研究。
登录
或
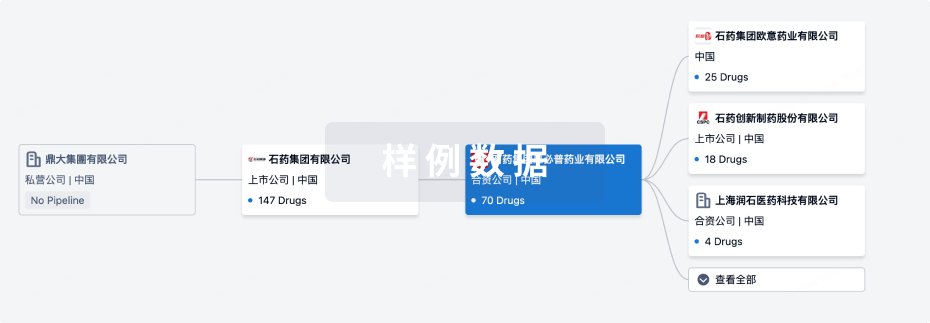
管线布局
2024年12月19日管线快照
管线布局中药物为当前组织机构及其子机构作为药物机构进行统计,早期临床1期并入临床1期,临床1/2期并入临床2期,临床2/3期并入临床3期
其他
2
登录后查看更多信息
当前项目
| 药物(靶点) | 适应症 | 全球最高研发状态 |
|---|---|---|
EGF Cancer Vaccine(In3Bio Research) | 非小细胞肺癌 更多 | 终止 |
表皮生长因子癌症疫苗(Center of Molecular Immunology) ( EGF ) | 非小细胞肺癌IIIB期 更多 | 终止 |
登录后查看更多信息
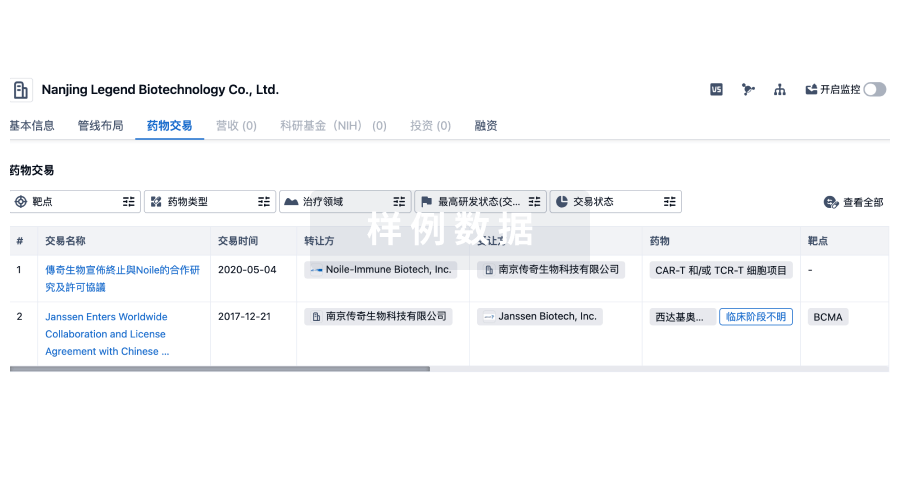
药物交易
使用我们的药物交易数据加速您的研究。
登录
或
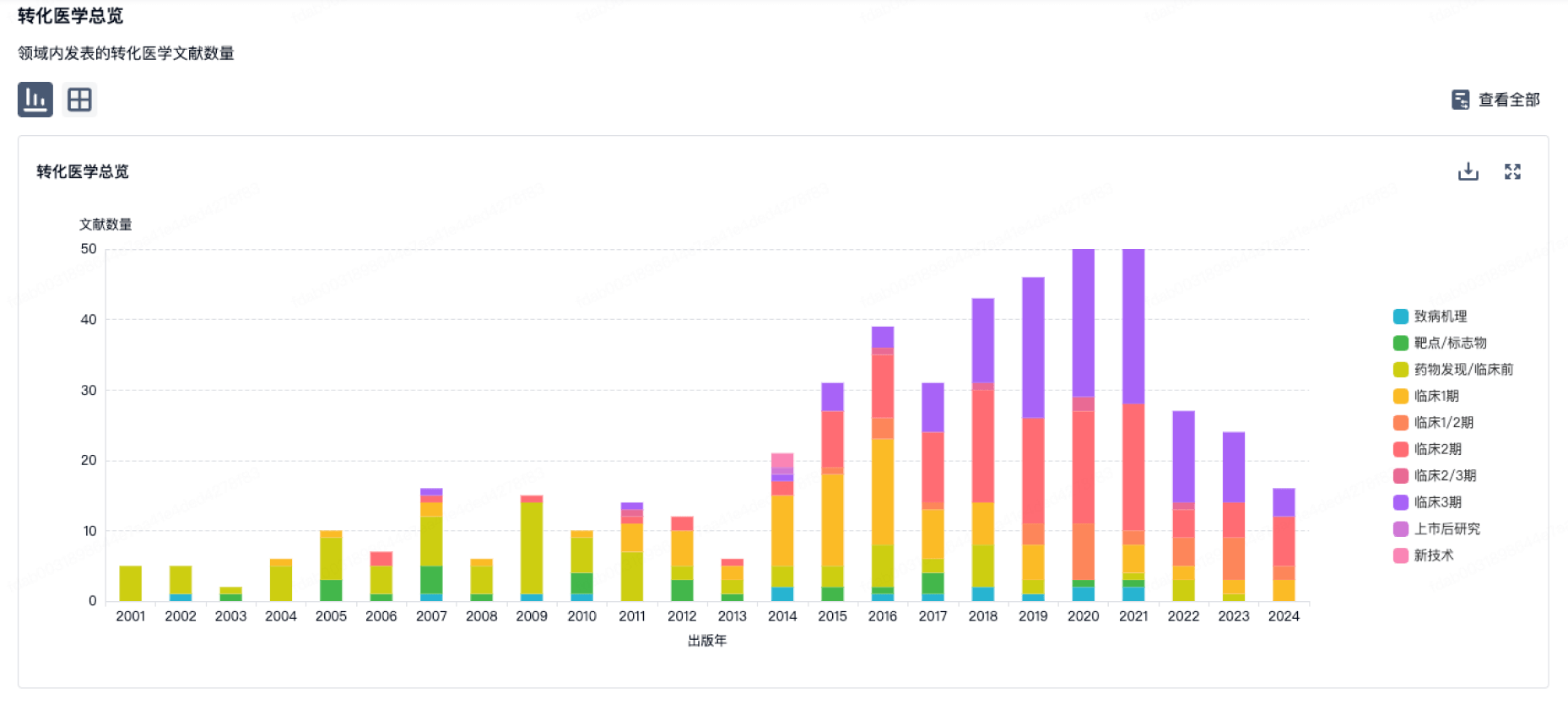
转化医学
使用我们的转化医学数据加速您的研究。
登录
或
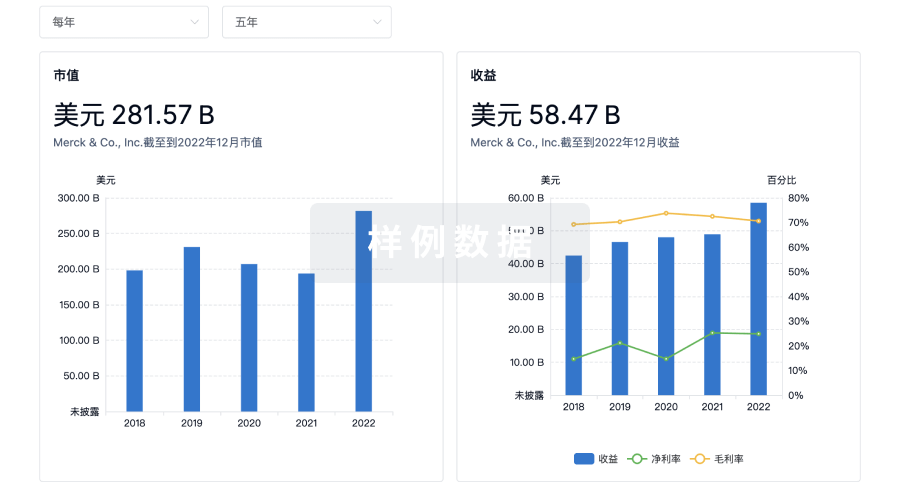
营收
使用 Synapse 探索超过 36 万个组织的财务状况。
登录
或
科研基金(NIH)
访问超过 200 万项资助和基金信息,以提升您的研究之旅。
登录
或
投资
深入了解从初创企业到成熟企业的最新公司投资动态。
登录
或
融资
发掘融资趋势以验证和推进您的投资机会。
登录
或
标准版
¥16800
元/账号/年
新药情报库 | 省钱又好用!
立即使用
来和芽仔聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用