The Effect of Protein Kinase C Inhibition on Renal and Peripheral Hemodynamic Function in Patients With Type 1 Diabetes Mellitus
Protein kinase C (PKC), an enzyme in the body, has been implicated in the process of diabetic microvascular complications. The purpose of this study will be to evaluate the renal hemodynamic and peripheral vascular effects of PKC inhibition with ruboxistaurin mesylate (an inhibitor of PKC) in patients with Type 1 diabetes mellitus and evidence of early nephropathy. In this pilot study, 21 patients with type 1 diabetes were planned to be randomized to LY333531 or placebo in a 2:1 fashion, after an initial period of testing. After 8 weeks of study drug, patients were retested.
Open-Label Treatment for Patients Completing Study B7A-MC-MBCM
To provide ruboxistaurin treatment to patients who completed the B7A-MC-MBCM study (NCT00604383), and who are felt by the investigator to have the potential to benefit from the ruboxistaurin treatment. Patients must be off study drug for 6 to 18 months from completion of B7A-MC-MBCM before beginning B7A-MC-MBDV. Additional data will be gathered to determine the long-term safety and effect of ruboxistaurin on vision.
The Effect of Ruboxistaurin on Vision Loss in Patients With Diabetes Mellitus and Clinically Significant Macular Edema
The purpose of the study is to test the hypothesis that oral administration of ruboxistaurin will reduce the occurrence of sustained moderate visual loss (SMVL) in patients with clinically significant macular edema. SMVL is defined as a 15 letter or more decrease from baseline in best-corrected Early Treatment Diabetic Retinopathy Study (ETDRS) visual acuity that is sustained for the patient's last 6 months of study participation. The SMVL data from this study will be combined with the SMVL data from Study B7A-MC-MBDL for the purpose of comparing ruboxistaurin to placebo.
100 项与 Chromaderm, Inc. 相关的临床结果
0 项与 Chromaderm, Inc. 相关的专利(医药)
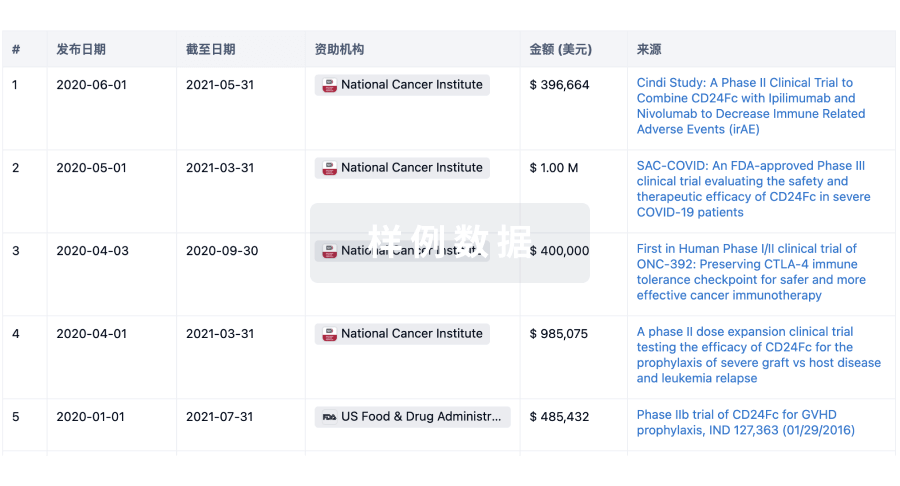
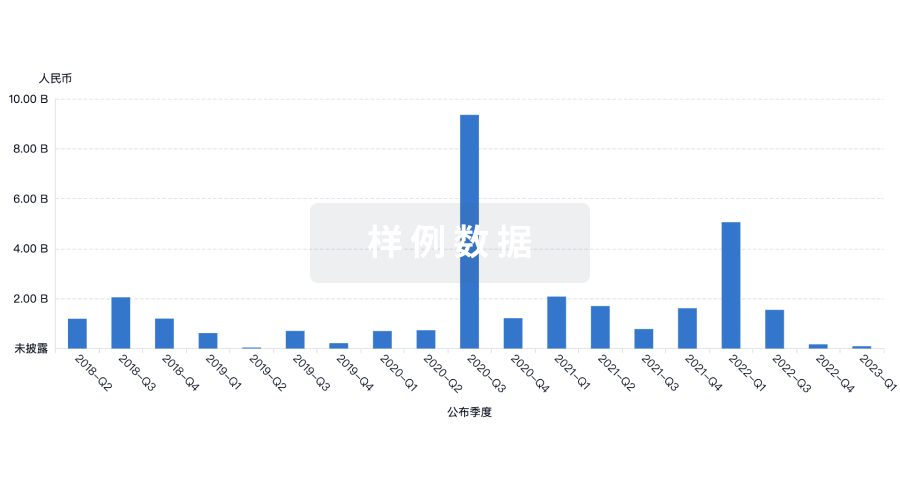
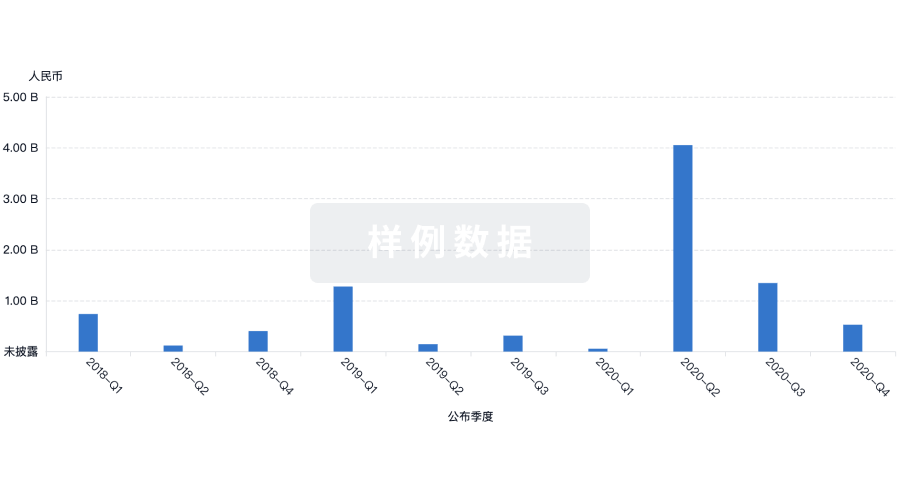
100 项与 Chromaderm, Inc. 相关的药物交易
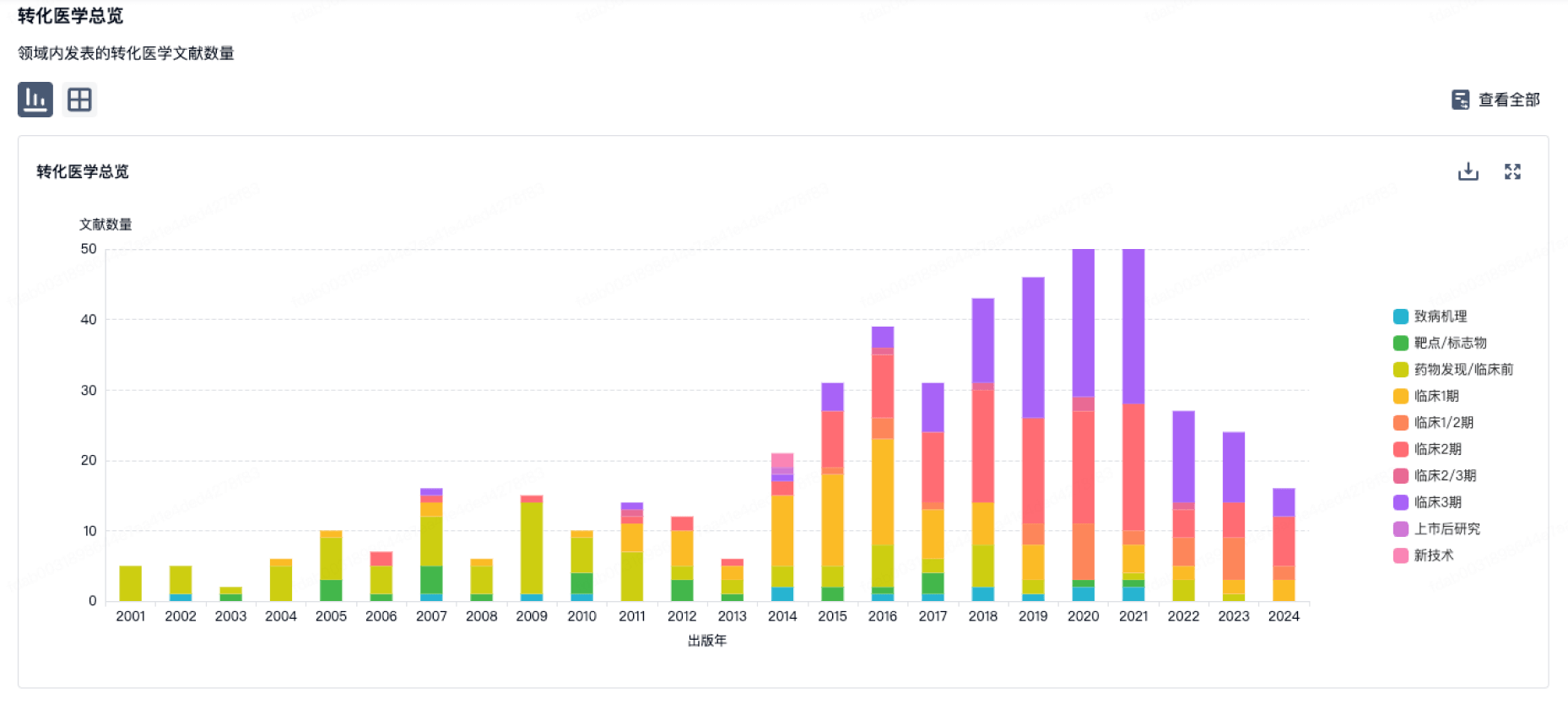
100 项与 Chromaderm, Inc. 相关的转化医学