靶点- |
作用机制- |
在研机构- |
|
在研适应症- |
|
最高研发阶段无进展 |
首次获批国家/地区- |
首次获批日期- |
|
|
在研机构- |
|
在研适应症- |
|
最高研发阶段无进展 |
首次获批国家/地区- |
首次获批日期- |
|
|
在研机构- |
|
在研适应症- |
|
最高研发阶段无进展 |
首次获批国家/地区- |
首次获批日期- |
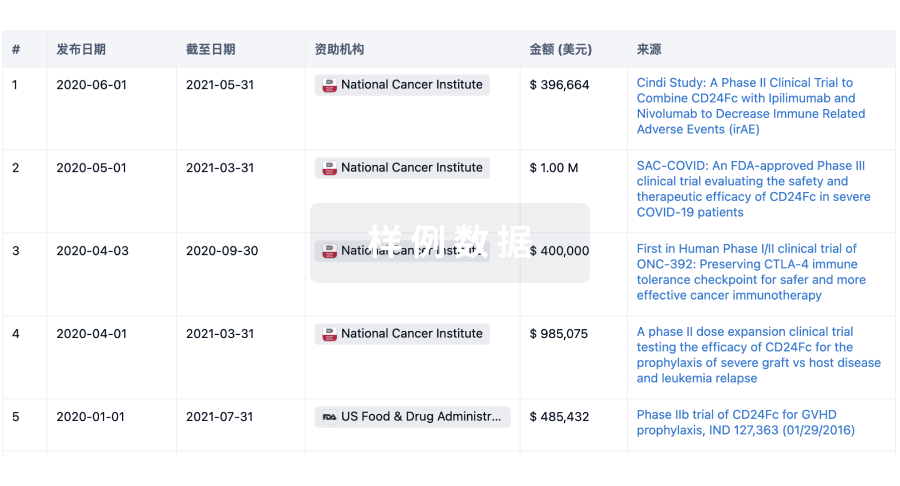
POC Study of Pipamperone 15mg Added to Stable Risperidone or Paliperidone Treatment in Chronic Schizophrenic and Schizoaffective Patients With Residual Symptoms: a Phase I/IIa, Randomized, Double-blind, Placebo-controlled Trial of 7 Weeks
This Phase I/IIa Proof-of-Concept (PoC) trial is designed to assess the effect of adding a single and repeated low dose (15mg/d) of pipamperone (PIP) for 6 weeks to stable treatment with an effective dose of risperidone (RIS) or paliperidone (PAL) on functional MRI tests and clinical outcome of chronic schizophrenic patients with residual, so-called 'positive' symptoms, as well as on cognition, motivation, subjective well-being of patients, negative symptoms, general psychopathological symptoms and safety/tolerability.
Pipamperone/Citalopram (PNB01) Versus Citalopram (CIT) and Versus Pipamperone (PIP) in the Treatment of Moderate to Severe Major Depressive Disorder (MDD): a Randomized, Double-blind Phase III Study of 10 Weeks
The overall objective of this trial is to demonstrate clinically relevant superior antidepressant efficacy of the fixed dose combination PNB01 (low dose pipamperone and citalopram) over reference antidepressant treatment with citalopram alone, and a low dose of psychoactive pipamperone alone in patients with moderate to severe Major Depressive Disorder.
This study was specifically designed to assess patient related outcome (PRO) parameters using an Interactive Voice Response System (IVRS) via telephone.
A Phase I, open label trial in healthy subjects to evaluate the central nervous receptor occupancy of pipamperone at the 5-HT2A and D2 receptor by means of Positron Emission Tomography. - Pipamperone PET study
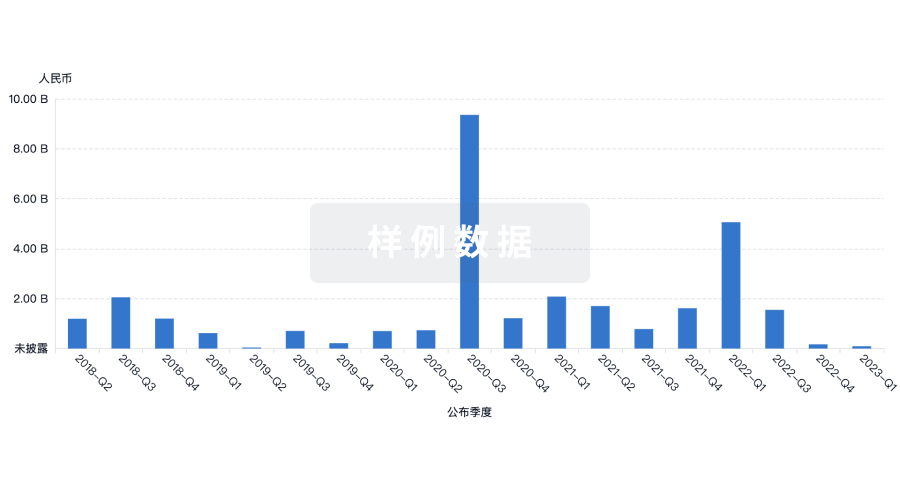
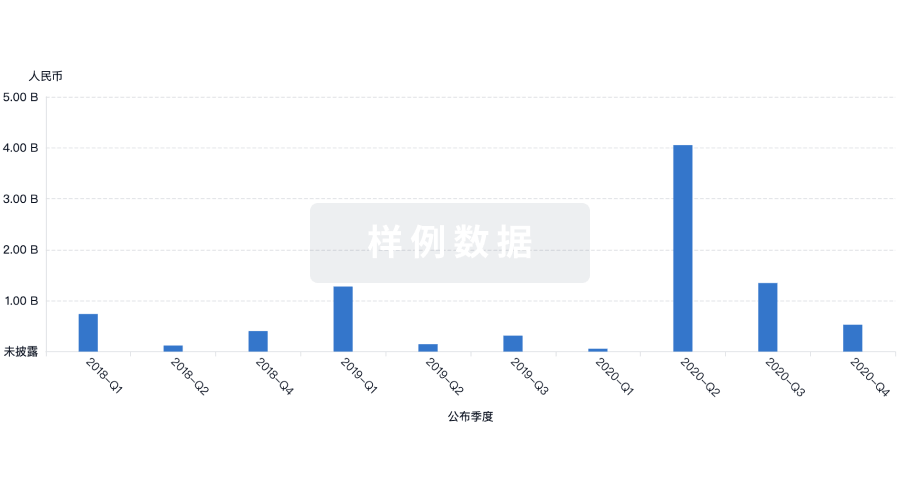
100 项与 PharmaNeuroBoost NV 相关的临床结果
0 项与 PharmaNeuroBoost NV 相关的专利(医药)
100 项与 PharmaNeuroBoost NV 相关的药物交易
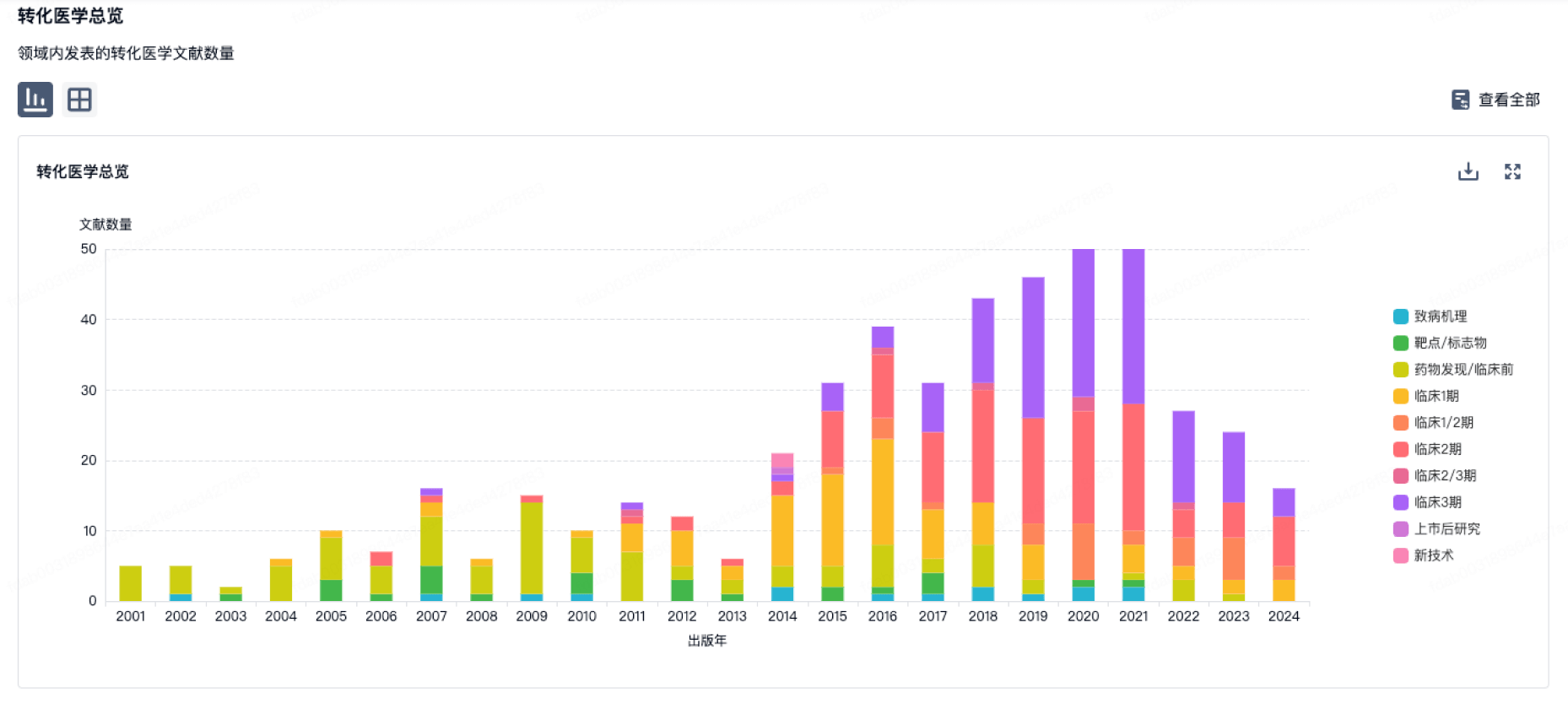
100 项与 PharmaNeuroBoost NV 相关的转化医学