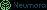FDA grants orphan drug status to NovelMed’s PNH treatment
2024-04-16
临床1期孤儿药
来源: Pharmaceutical Technology
Breakdown of PNH RBCs within and outside the blood vessels. Credit: Phonlamai Photo / Shutterstock.com.
NovelMed has announced the receipt of orphan drug designation (ODD) from the US Food and Drug Administration (FDA) for its investigational drug, NM5072, for treating paroxysmal nocturnal hemoglobinuria (PNH).
An alternative pathway blocker anti-properdin antibody, NM5072 selectively hinders the immune system components responsible for the disease, inhibiting alternative pathway cascades and preventing the lysis of PNH red blood cells (RBCs).
The drug has concluded a Phase I clinical trial in 48 healthy volunteers with a good safety profile.
It is currently being reviewed for multiple indications by regulators in the US and internationally.
See Also:
Qureight secures Series A funding to accelerate drug development

Preview
来源: Pharmaceutical Technology
FDA halts Neumora’s schizophrenia drug trial over convulsions in rabbits

Preview
来源: Pharmaceutical Technology
PNH is a rare disease characterised by chronic inflammation and haemolysis, in which PNH RBCs break down within and outside blood vessels, leading to anaemia and severe health implications.
“We are hopeful that NM5072, with its unique mechanism of action that targets the top of the complement cascade, could become a promising treatment to improve outcomes in these patients.”
The FDA’s ODD provides benefits including seven years of market exclusivity upon the receipt of approval, tax credits for clinical trial costs, fee waivers, reduced annual product fees, assistance for clinical protocol development and the possibility of expedited development programmes.
更多内容,请访问原始网站
文中所述内容并不反映新药情报库及其所属公司任何意见及观点,如有版权侵扰或错误之处,请及时联系我们,我们会在24小时内配合处理。
适应症
靶点
-药物
热门报告
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。