After EQRx partnership falls apart, Lynk sticks the landing in midstage eczema trial
2023-08-09
临床2期临床结果引进/卖出并购临床3期
Preview
来源: FierceBiotech
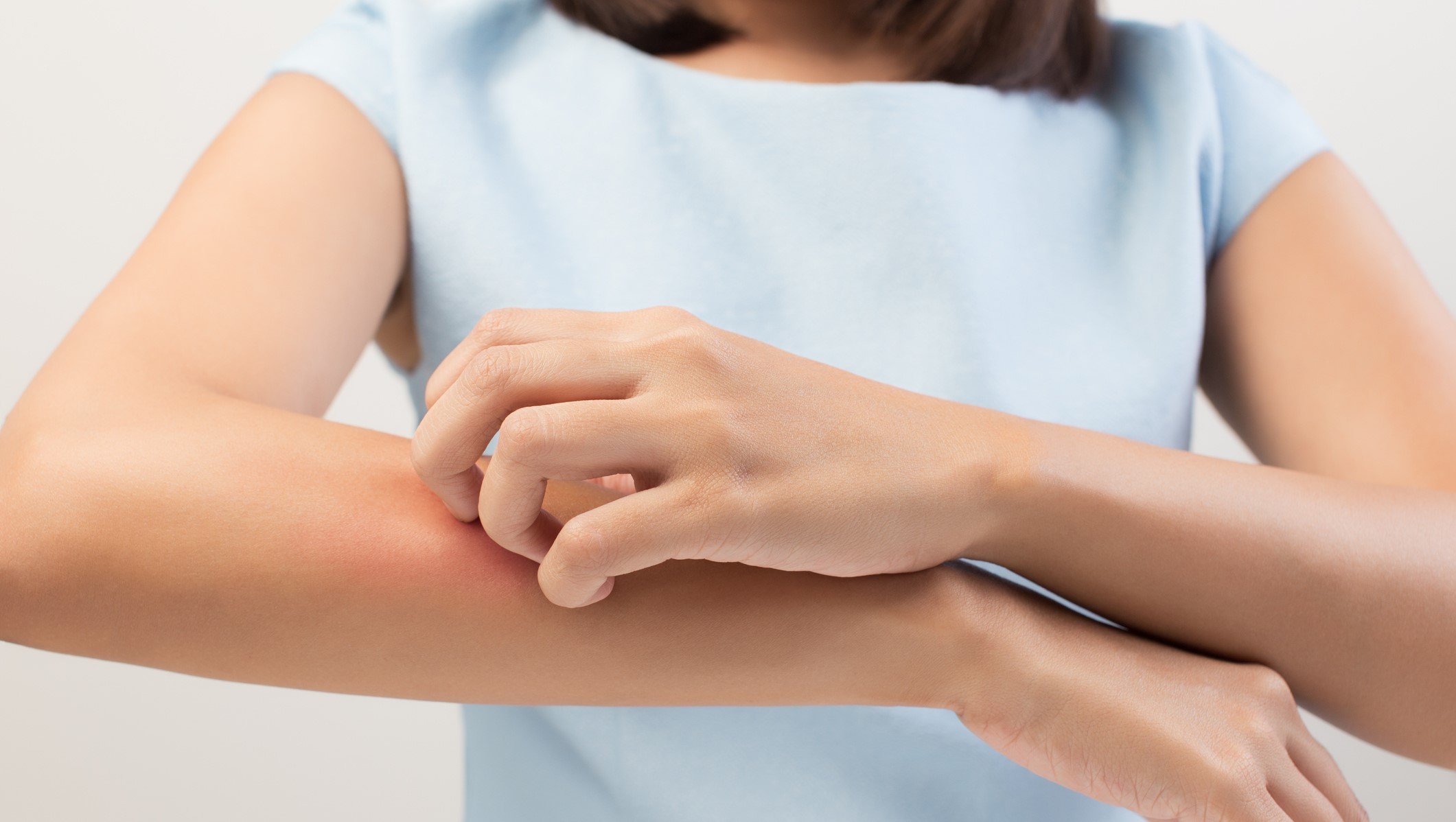
Lynk Pharmaceuticals found that eczema trial participants had significant improvement in skin itchiness compared to placebo.
Part of EQRx’s implosion earlier this year included cutting ties with China-based Lynk Pharmaceuticals. However, Lynk seems to have safely removed itself from EQRx’s wreckage, as its eczema asset improved area and severity in a phase 2 clinical trial.
The JAK1 inhibitor LNK01001 significantly improved eczema area and severity at 12 weeks of treatment, according to Lynk's Aug. 9 release. The phase 2, randomized trial enrolled 150 adults with moderate to severe eczema who had previously experienced an inadequate response to topical treatment or previously received other systemic treatment for eczema. Trial participants were divided into three trial groups: one receiving a high-dose of LNK01001, one with a low-dose and one placebo group.
The primary efficacy endpoint was percent change from baseline at 12 weeks, as measured by an industry-standard scale. While Lynk didn’t share specific data points, it said patients in both treatment groups showed statistically significant improvement, hitting the study’s main goal.
The trial also measured pruritus—a term for itchy skin—and found that both the high- and low-dose groups showed significant improvement in pruritus indices than the placebo group 24 hours after dosing, according to Lynk.
Both high and low-dose groups had good overall safety and tolerability profiles, with comparable rates of treatment-emergent adverse events and serious adverse events to the placebo group, the company said. No major adverse cardiovascular events, venous thromboembolism, malignancies or severe infections were reported.
Lynk is now looking to launch a phase 3 trial for the asset, according to the company release.
In early 2022, Lynk inked a partnership with China-based Simcere Pharmaceutical for the JAK1 inhibitor. Under the terms of the deal, Lynk is responsible for the development of LNK01001, while Simcere will have exclusive marketing rights in China for rheumatoid arthritis and ankylosing spondylitis indications.
While that collaboration is still intact, Lynk lost its partnership with serial biotech founder Alexis Borisy’s EQRx in May when the company dropped deals with both Lynk and CStone Pharmaceuticals. The Lynk deal, penned in 2020, gave EQRx exclusive licensing rights to JAK-1 inhibitorJAK-1 inhibitor LNK-207, also known as EQ121. The U.S. biotech gained global rights to the asset except in mainland China, Hong Kong, Macau and Taiwan, paying an undisclosed upfront fee giving Lynk the chance to make up to $172 million in milestone payments in return.
Alongside the severed ties, EQRx abandoned its plan to provide drugs that are typically expensive at lower prices. The biotech recently entered into an acquisition deal with Revolution Medicines—another of the Borisy's startups. Revolution has stated that it doesn’t intend to continue any of EQRX’s R&D work.
更多内容,请访问原始网站
文中所述内容并不反映新药情报库及其所属公司任何意见及观点,如有版权侵扰或错误之处,请及时联系我们,我们会在24小时内配合处理。
靶点
热门报告
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。



