ESMO 2022: Interim Results for Treatment of Platinum-Resistant Ovarian Cancer with p62 Plasmid DNA Show Statistically Significant Changes in Progression-Free Survival
2022-09-09
合作财报IPO
While the disease has progressed in all patients receiving standard of care, it did not progress in 40% of the patients receiving standard chemotherapy together with the experimental drug, Elenagen — and for half of the patients in the group, Elenagen has increased the time to disease progression by more than 2.5 times
BOSTON, Sept. 9, 2022 /PRNewswire/ -- CureLab Oncology, a clinical-stage, pre-IPO biotech company, is presenting the results of its ongoing ex-US clinical trial at this year's meeting of the European Society for Medical Oncology (ESMO). The trial is assessing an experimental DNA plasmid encoding the human protein, p62 (affectionately codenamed Elenagen), at the N.N. Alexandrov National Cancer Centre, one of the leading cancer centers in Eastern Europe.
Continue Reading
Preview
来源: PRNewswire
CureLab Oncology, a clinical-stage, pre-IPO biotech company, is presenting the results of its ongoing ex-US clinical trial at this year’s meeting of the European Society for Medical Oncology (ESMO).
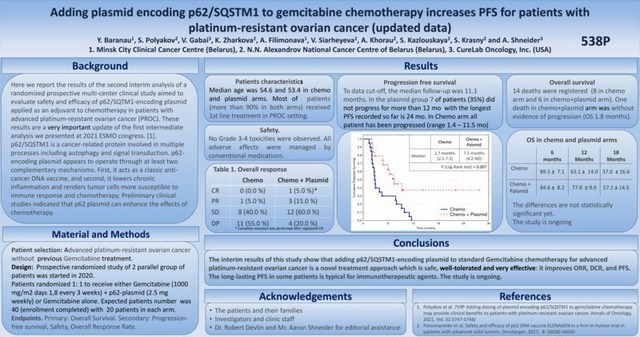
Platinum-resistant ovarian cancer (PROC) is one of the deadliest forms of ovarian cancer and has a very poor prognosis. The FDA approved other novel treatments for PROC based on statistically significant improvements for the delay of progression of the cancer, assessed by the increasing size of a primary tumor and/or the appearance of new metastatic lesions or growth of the existing ones; i.e., progression-free survival (PFS). A statistically significant prolongation of PFS was clearly demonstrated in the trial and continues to extend as the trial continues.
Summary of what CureLab Oncology is presenting at ESMO 2022
In the ex-US clinical trial, CureLab Oncology is assessing its experimental plasmid DNA therapeutic, Elenagen, for its ability to extend progression-free survival (PFS) in patients with PROC receiving standard chemotherapy, gemcitabine.
For all patients in the trial receiving the standard of care only, the disease progressed during the first year of observations, with 50% of patients progressing within 2.7 months.
In contrast, the disease did not progress at all for almost 40% of patients receiving the same gemcitabine in combination with weekly intramuscular injections of Elenagen.
In 50% of patients receiving standard of care only, the disease has progressed within 2.7 months, while the time to progression for 50% of patients in the "chemo plus Elenagen" group has increased to 7.2 months.
To date, no patient receiving Elenagen demonstrated any adverse event (AE) or significant adverse event (SAE) greater than grade 1, and all resolved quickly without impact.
So far, the longest PFS in the gemcitabine + Elenagen treatment group is 27 months (and continues to grow), while the disease has progressed in all patients in the gemcitabine-only group within less than 12 months.
"While our data on increasing progression-free survival are already statistically significant (P=0.01), it will take a little longer to reach a statistical significance on overall survival of the patients," said Professor Sergey Krasny, M.D., D.Sc., co-author of the ESMO presentation, and a medical director of the study. "However, so far, no single patient receiving gemcitabine alone has lived longer than 18 months, while over 50 percent of patients receiving gemcitabine and Elenagen live longer than that. Also of note is that no person who survived to live 18 months has died at this point in the study."
"Our presentation at ESMO 2022 is a great step forward compared to what we presented at ESMO 2021. Now, we are certain that Elenagen is increasing progression-free survival for platinum-resistant ovarian cancer patients in the trial," said Alexander Shneider, Ph.D., CEO of CureLab Oncology.
About Elenagen
CureLab's lead investigational compound is code-named Elenagen, an experimental DNA therapy that consists of a circular piece of DNA called a plasmid that includes a gene for a human protein called p62/SQSTM1. In animal studies and Phase I/II human trials conducted ex-US, Elenagen demonstrated promise in reversing tumor grade, changing the tumor microenvironment, and enhancing the anti-cancer effects of chemotherapy. Experimental results also indicate mitigation of chronic inflammation and stimulation of an immune response to the tumor.
About CureLab Oncology
CureLab Oncology Inc. is a pre-IPO, clinical-stage immuno-oncology biotech company headquartered in the greater Boston area, Massachusetts. CureLab is dedicated to advancing new and safer therapeutics for solid tumors and other oncology and inflammatory indications. To learn more, visit curelab.com.
Media contact:
Tim Cox, ZingPR, [email protected]
SOURCE CureLab Oncology Inc.
更多内容,请访问原始网站
文中所述内容并不反映新药情报库及其所属公司任何意见及观点,如有版权侵扰或错误之处,请及时联系我们,我们会在24小时内配合处理。
靶点
热门报告
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。


