更新于:2024-11-11
Licaminlimab
更新于:2024-11-11
概要
基本信息
序列信息
Sequence Code 315605447

来源: *****
关联
3
项与 Licaminlimab 相关的临床试验A Multi-center, Randomized, Double-Masked, Vehicle-Controlled Study Evaluating the Efficacy and Safety of Licaminlimab for the Treatment of Dry Eye Disease
The goal of this clinical trial is to learn about licaminlimab (OCS-02) in the treatment of dry eye disease. The main question it aims to answer is if licaminlimab ophthalmic suspension is more effective than vehicle in treating signs of dry eye disease.
开始日期2023-11-29 |
申办/合作机构 Oculis SA [+1] |
A Multicenter, Randomized, Double-Masked, Active-Controlled Study to Evaluate the Safety and Efficacy of LME636 in Patients With Acute Anterior Uveitis
The purpose of the study is to determine whether topical ocular administration of LME636 60 mg/mL is efficacious in resolving the ocular inflammation in the anterior chamber (AC) associated with acute anterior uveitis (AAU).
开始日期2015-07-17 |
申办/合作机构 |
A Randomized, Double-masked, Vehicle-controlled Study of LME636 in the Relief of Persistent Ocular Discomfort in Subjects With Severe Dry Eye Disease
The purpose of this study is to evaluate the efficacy of LME636 compared to vehicle in the reduction of ocular symptoms and to evaluate the safety and tolerability of LME636, when administered topically for up to 42 days, in subjects with severe dry eye disease.
开始日期2015-03-09 |
申办/合作机构  Alcon AG Alcon AG [+1] |
100 项与 Licaminlimab 相关的临床结果
登录后查看更多信息
100 项与 Licaminlimab 相关的转化医学
登录后查看更多信息
100 项与 Licaminlimab 相关的专利(医药)
登录后查看更多信息
9
项与 Licaminlimab 相关的文献(医药)2025-01-01·JOURNAL OF ETHNOPHARMACOLOGY
Evidence to support cultivated fruiting body of Ophiocordyceps sinensis (Ascomycota)'s role in relaxing airway smooth muscle
Article
ETHNOPHARMACOLOGICAL RELEVANCE:
Ophiocordyceps sinensis (O. sinensis) is a genus of Ascomycete fungus that is endemic to the alpine meadows of the Tibetan Plateau and adjoining Himalayas. It has been used traditionally as a tonic to improve respiratory health in ancient China as well as to promote vitality and longevity. Bioactive components found in O. sinensis such as adenosine, cordycepin, 3-deoxyadenosine, L-arginine and polysaccharides have gained increasing interest in recent years due to their antioxidative and other properties, which include anti-asthmatic, antiviral, immunomodulation and improvement of general health.
AIM OF THE STUDY:
This study's primary aim was to investigate the effect of a cultivated fruiting body of O. sinensis strain (OCS02®) on airways patency and the secondary focus was to investigate its effect on the lifespan of Caenorhabditis elegans.
MATERIALS AND METHODS:
A cultivated strain, OCS02®, was employed and the metabolic profile of its cold-water extract (CWE) was analysed through liquid chromatography-mass spectrometry (LC-MS). Organ bath approach was used to investigate the pharmacological properties of OCS02® CWE when applied on airway tissues obtained from adult male Sprague-Dawley rats. The airway relaxation mechanisms of OCS02® CWE were explored using pharmacological tools, where the key regulators in airway relaxation and constriction were investigated. For the longevity study, age-synchronised, pos-1 RNAi-treated wild-type type Caenorhabditis elegans at the L4 stage were utilised for a lifespan assay.
RESULTS:
Various glycopeptides and amino acids, particularly a high concentration of L-arginine, were identified from the LC-MS analysis. In airway tissues, OCS02® CWE induced a significantly greater concentration-dependent relaxation when compared to salbutamol. The relaxation response was significantly attenuated in the presence of NG-Nitro-L-arginine methyl ester (L-NAME), 1H-[1,2,4]oxadiazolo [4,3-a]quinoxalin-1-one (ODQ) and several K+ channel blockers. The longevity effect induced by OCS02® CWE (5 mg/mL and above) was observed in C. elegans by at least 17%.
CONCLUSIONS:
These findings suggest that the airway relaxation mechanisms of OCS02® CWE involved cGMP-dependent and cGMP-independent nitric oxide signalling pathways. This study provides evidence that the cultivated strain of OCS02® exhibits airway relaxation effects which supports the traditional use of its wild O. sinensis in strengthening respiratory health.
2024-10-01·Cornea
Pharmacogenomic Analysis of Response to Topical Tumor Necrosis Factor α Antagonist Licaminlimab (OCS-02) in Dry Eye Disease [RETRACTED]
Article
作者: Baudouin, Christophe ; Galor, Anat ; He, Yunsheng ; Weissgerber, Georges ; Perez, Victor L. ; Donnenfeld, Eric
Purpose::
The purpose of this study was to evaluate the pharmacogenomics of response to topical ocular tumor necrosis factor α (TNFα) inhibitor licaminlimab in patients with DED.
Methods::
Three single-nucleotide polymorphisms (SNPs) associated with Sjögren syndrome, 3 in the TNFα gene and 1 in the TNF receptor 1 (TNFR1) gene, were assessed for association with response to licaminlimab in participants from a randomized, vehicle-controlled, Phase 2 study in which adults with DED and severe ocular discomfort persisting despite treatment with artificial tears received licaminlimab or vehicle for 6 weeks. Response was assessed for change from baseline in Global Ocular Discomfort score at Day 29 of treatment. The pharmacogenomic analysis was a prospectively specified exploratory objective of the study. mRNA expression for TNFα, interleukin (IL) 1β, and IL8 in conjunctival epithelium cells was determined. The relationship between SNPs and response to licaminlimab was assessed using a mixed model repeated measures analysis.
Results::
SNP rs1800693 in the TNFR1 gene showed a significant effect on response to licaminlimab (P < 0.0001, initial association test); no effect was seen for any of the other SNPs tested. The CC genotype of rs1800693 was associated with much greater response to licaminlimab than the CT or TT genotypes: LS mean changes from baseline to Day 29 in Global Ocular Discomfort score were −29.5, −0.09, and −3.90, in patients with the CC, CT, and TT genotypes, respectively (P < 0.0001). No significant effect was observed in vehicle-treated patients. Improvements from baseline were seen in 3/4 licaminlimab-treated participants with the CC genotype. Conjunctival epithelium cell levels of mRNA for TNFα, IL1β, and IL8 decreased from baseline in participants with the CC genotype, but not with the CT or TT genotypes. Between-genotype differences in mRNA levels were not observed in participants receiving vehicle.
Conclusions::
The CC genotype of rs1800693, relatively common in patients with DED, was strongly associated with response to licaminlimab and decreased inflammatory cytokine gene expression in ocular surface cells during treatment. This study is one of the first to our knowledge to investigate pharmacogenomics in the treatment of DED.
2024-10-01·CORNEA
Pharmacogenomic Analysis of Response to Topical Tumor Necrosis Factor α Antagonist Licaminlimab (OCS-02) in Dry Eye Disease: Retraction
Article
74
项与 Licaminlimab 相关的新闻(医药)2024-11-07
ZUG, Switzerland, Nov. 07, 2024 (GLOBE NEWSWIRE) --
Significant advancement on product portfolio, including enrollment in the OCS-01 DIAMOND Phase 3 program in DME and OCS-05 Phase 2 ACUITY trial in acute optic neuritis (AON) with topline readout anticipated in December 2024Leadership team bolstered with extensive experience in key areas as the Company advances its late-stage pipeline and prepares for commercial phaseCash, cash equivalents and short-term investments of $125.0 million as of September 30, 2024, provides cash runway into 2H 2026
Oculis Holding AG (Nasdaq: OCS; XICE: OCS) (“Oculis” or the “Company”), a global biopharmaceutical company purposefully driven to save sight and improve eye care, today announced results for the quarter ended September 30, 2024, and provided an overview of the Company’s progress.
Riad Sherif M.D., Chief Executive Officer of Oculis: “During the quarter, we achieved excellent momentum in product pipeline development. We continue to accelerate recruitment for both Phase 3 trials in our core DIAMOND program with OCS-01 in DME and expanded this program’s committees with several world-renowned retina experts. Looking ahead, we are excited for the upcoming topline readout from the OCS-05 Phase 2 ACUITY trial in AON, anticipated in December 2024. The results will provide us with meaningful insights about the safety and tolerability of OCS-05, and its potential as a neuroprotective candidate in acute optic neuritis and other neuro-ophthalmic diseases. With a strengthened leadership team including recent appointments of Sharon Klier, M.D. as Chief Development Officer and Daniel S. Char as Chief Legal Officer, and a solid balance sheet, Oculis is well positioned to drive execution in pipeline development and create value for key stakeholders.”
Q3 2024 and Recent Highlights
Clinical Highlights:
Substantial enrollment progress was achieved since the end of Q2 2024 through early October, with ~70% of patients enrolled in the Phase 3 DIAMOND-1 trial, and ~40% of patients enrolled in the Phase 3 DIAMOND-2 trial.Expanded DIAMOND program committees with leading retina experts announced for the Phase 3 program of OCS-01 in DME.
Presentations and Awards Highlights:
David Eichenbaum, M.D., presented an update on the DIAMOND Phase 3 program with OCS-01, an OPTIREACH® formulation of high concentration dexamethasone eye drop, for DME at Innovate Retina, an event that focuses exclusively on game-changing innovations in medical and surgical retina care. His presentation highlighted the potential of OCS-01 to become the first non-invasive therapy for DME to address unmet medical needs for early treatment intervention and for patients inadequately controlled with current therapies.At the 2024 EURETINA congress, the inaugural Ramin Tadayoni Award, supported by Oculis, was awarded to Andrea Govetto, M.D., Ph.D. who is developing a computational model of fluid flow and retinal tissue deformation in macular edema. The Ramin Tadayoni Award, awarded by EURETINA, was established in partnership with EURETINA in memory of Oculis’ Chief Scientific Officer, EURETINA past President, and a world-renowned retina specialist in order to pay a lasting tribute to the legacy of Professor Tadayoni, who passed away unexpectedly earlier this year.
Company Updates and Upcoming Milestones
OCS-01: Following a pre-NDA meeting with the U.S. Food and Drug Administration (FDA) in August 2024 for post-operative pain and inflammation, the Company plans to be NDA submission ready in Q1 2025.OCS-02: The Company is planning to consult with the FDA in Q1 2025 to discuss the positive topline results from the Phase 2b RELIEF trial and next steps for OCS-02 (licaminlimab) development. If approved, OCS-02 (licaminlimab) has the potential to be the first precision medicine for DED given the predictive and more pronounced effects observed in a specific TNFR1 genotype population.OCS-05: Topline readout for the Phase 2 ACUITY trial in AON is on track for December 2024. OCS-05 is a peptidomimetic serum glucocorticoid kinase-2 (SGK-2) activator, a novel mechanism of action, with the potential to be a neuroprotective therapy for neuro-ophthalmic diseases.OCS-05 is being evaluated for the treatment of AON in the ACUITY Phase 2 trial, a randomized, double-blind, placebo-controlled, multi-center trial in France, designed to evaluate the safety and tolerability of OCS-05. Enrollment is complete with 36 patients randomized. The primary endpoint is safety, and additional exploratory measurements will be evaluated to explore the potential neuroprotective benefits of OCS-05 in AON patients.AON is a rare disease of an acute inflammation of the optic nerve that can lead to permanent visual impairment. AON mainly occurs in 20- to 40-year-old adults and affects up to 8 in 100,000 people worldwide1. While corticosteroids are used to treat the inflammation, there remains a critical unmet medical need for therapies that preserve vision or provide neuroprotection after an acute episode of optic neuritis.In animal models of neuroinflammation and neurodegeneration, OCS-05 has shown evidence of neuroprotective activity, including the prevention of retinal ganglion cell damage in glaucoma and AON models, and promotion of axonal sparing and reduction of demyelination in AON model.There were no drug-related side effects with OCS-05 reported from the Phase 1 randomized, double-blind, placebo-controlled, single and multiple ascending dose trial that was completed in 48 healthy adult volunteers (36 on OCS-05, 12 on placebo) in the U.K.
Q3 2024 Financial Highlights
Cash position: As of September 30, 2024, the Company had total cash, cash equivalents and short-term investments of CHF 105.5 million or $125.0 million, compared to CHF 91.7 million or $109.0 million as of December 31, 2023. The increase in cash position from December 31, 2023 reflects proceeds from the registered direct offering in the second quarter of 2024. Based on its current development plans, the Company’s cash balances are expected to fund operations into the second half of 2026.Research and development expenses were CHF 13.0 million or $15.0 million for the three-months ended September 30, 2024, compared to CHF 8.9 million or $10.0 million in the same period in 2023. The increase was primarily due to higher clinical trial expenses in the ongoing OCS-01 DIAMOND Stage 2 trials and OCS-05 ACUITY trial.General and administrative expenses were CHF 5.3 million or $6.2 million for the three-months ended September 30, 2024, compared to CHF 4.3 million or $4.9 million in the same period in 2023. The increase was primarily due to stock-based compensation expenses.Q3 Quarter-to-date Net loss was CHF 20.2 million or $23.3 million for the third quarter ended September 30, 2024, compared to CHF 17.4 million or $19.7 million for the same period in 2023. The increase was primarily driven by increases in OCS-01 clinical development related expenses.Q3 Year-to-date net loss was CHF 57.1 million or $64.8 million for the nine months ended September 30, 2024, compared to CHF 76.3 million or $84.5 million for the same period in 2023. The decrease was primarily due to a non-recurring and non-cash merger and listing expense recorded in 2023 of CHF 34.9 million or $38.2 million, partially offset by increases in clinical development costs and expenses incurred to operate as a public company.Q3 Year-to-date Non-IFRS net loss was CHF 57.1 million or $64.8 million, or CHF 1.44 or $1.63 per share, for the nine months ended September 30, 2024, compared to CHF 36.5 million or $40.4 million, or CHF 1.32 or $1.46 per share, for the same period in 2023. The increase in non-IFRS net loss was primarily driven by advances of clinical development programs.
Non-IFRS Financial Information
This press release contains financial measures that do not comply with International Financial Reporting Standards (IFRS) including non-IFRS loss, and non-IFRS loss attributable to equity holders per common share. These non-IFRS financial measures exclude the impact of items that the Company’s management believes affect comparability or underlying business trends. These measures supplement the Company’s financial results prepared in accordance with IFRS. The Company’s management uses these measures to better analyze its financial results and better estimate its financial outlook. In management’s opinion, these non-IFRS measures are useful to investors and other users of the Company's financial statements by providing greater transparency into the ongoing operating performance of the Company and its future outlook. Such measures should not be deemed to be an alternative to IFRS requirements.
The non-IFRS measures for the reported periods reflect adjustments made to exclude:
Merger and listing expense, which was a one-time non-cash expense CHF 34.9 million or $38.2 million in total operating expenses in the nine months ended September 30, 2023.During the third quarter of 2023, the Company gave effect to the impending dissolution of its Merger Sub 2 entity pursuant to the Business Combination Agreement with EBAC, which was ultimately completed in April 2024. As a result, the cumulative translation adjustments related to Merger Sub 2 previously reported in equity and recognized in other comprehensive loss, were reclassified from equity to the Condensed Consolidated Interim Statement of Loss for the three and nine months ended September 30, 2023. The resulting non-cash foreign exchange impact of such reclassification amounted to CHF 5.0 million or $5.7 million for the three and nine months ended September 30, 2023.
Condensed Consolidated Statements of Financial Position (Unaudited) (Amounts in CHF thousands)As of September 30, As of December 31, 2024 2023ASSETS
Non-current assets Property and equipment, net366 288Intangible assets12.206 12.206Right-of-use assets1.386 755Other non-current assets159 89Total non-current assets14.117 13.338 Current assets Other current assets4.450 8.488Accrued income1.568 876Short-term financial assets69.841 53.324Cash and cash equivalents35.632 38.327Total current assets111.491 101.015 TOTAL ASSETS125.608 114.353 EQUITY AND LIABILITIES
Shareholders' equity Share capital429 366Share premium340.645 288.162Reserve for share-based payment13.319 6.379Actuarial loss on post-employment benefit obligations (1.919) (1.072)Treasury shares (10) -Cumulative translation adjustments (334) (327)Accumulated losses (256.902) (199.780)Total equity95.228 93.728 Non-current liabilities Long-term lease liabilities929 431Long-term payables- 378Defined benefit pension liabilities1.734 728Total non-current liabilities2.663 1.537 Current liabilities Trade payables4.892 7.596Accrued expenses and other payables14.704 5.948Short-term lease liabilities314 174Warrant liabilities7.807 5.370Total current liabilities27.717 19.088 Total liabilities30.380 20.625 TOTAL EQUITY AND LIABILITIES125.608 114.353
Condensed Consolidated Statements of Loss (Unaudited) (Amounts in CHF thousands, except per share data) For the three months ended September 30, For the nine months ended September 30, 2024 2023 2024 2023Grant income 216 219 683 698Operating income 216 219 683 698Research and development expenses (12.999) (8.872) (40.320) (21.218)General and administrative expenses (5.348) (4.306) (16.307) (13.147)Merger and listing expense - - - (34.863)Operating expenses (18.347) (13.178) (56.627) (69.228)
Operating loss (18.131) (12.959) (55.944) (68.530)
Finance income 556 520 1.797 773Finance expense (264) (11) (392) (1.303)Fair value adjustment on warrant liabilities (445) (2.434) (2.144) (4.638)Foreign currency exchange gain (loss), net (1.888) (2.645) (361) (2.485)Finance result, net (2.041) (4.570) (1.100) (7.653)
Loss before tax for the period (20.172) (17.529) (57.044) (76.183)
Income tax expense (18) 116 (78) (120)
Loss for the period (20.190) (17.413) (57.122) (76.303)
Loss per share:
Basic and diluted loss attributable to equity holders
(0,48)
(0,48)
(1,44)
(2,76)
Reconciliation of Non-IFRS Measures (Unaudited) (Amounts in CHF thousands, except per share data)
For the three months ended September 30,For the nine months ended September 30, 2024 2023 2024 2023 IFRS loss for the period (20.190) (17.413) (57.122) (76.303) Non-IFRS adjustments:
Merger and listing expense (i)- - - 34.863 Merger Sub 2 reclassification from equity to foreign exchange loss (ii) 4.978 4.978 Non-IFRS loss for the period (20.190) (12.435) (57.122) (36.462)
IFRS basic and diluted loss attributable to equity holders
(0,48)
(0,48)
(1,44)
(2,76) Non-IFRS basic and diluted loss attributable to equity holders
(0,48)
(0,34)
(1,44)
(1,32)
IFRS weighted-average number of shares used to compute loss per share basic and diluted41.807.918 36.330.836 39.659.305 27.673.950
(i) Merger and listing expense is the difference between the fair value of the shares transferred and the fair value of the EBAC net assets per the Business Combination Agreement. This merger and listing expense is non-recurring in nature and represented a share-based payment made in exchange for a listing service and does not lead to any cash outflows. (ii) The reclassification of cumulative translation adjustments from equity to foreign exchange loss results from the impact of the impending dissolution of Merger Sub 2, which is expected to occur in the coming months. This exchange loss is non-recurring in nature and does not lead to any cash outflows.
-ENDS-
About Oculis
Oculis is a global biopharmaceutical company (Nasdaq: OCS; XICE: OCS) purposefully driven to save sight and improve eye care. Oculis’ highly differentiated pipeline comprises multiple innovative product candidates in development. It includes OCS-01, a topical eye drop candidate for diabetic macular edema (DME) and for the treatment of inflammation and pain following cataract surgery; OCS-02 (licaminlimab), a topical biologic anti-TNFα eye drop candidate for dry eye disease (DED) and for non-infectious anterior uveitis; and OCS-05, a neuroprotective candidate for acute optic neuritis (AON). Headquartered in Switzerland and with operations in the U.S. and Iceland, Oculis’ goal is to improve the health and quality of life of patients worldwide. The company is led by an experienced management team with a successful track record and is supported by leading international healthcare investors.
For more information, please visit: www.oculis.com
Oculis Contacts
Ms. Sylvia Cheung, CFOsylvia.cheung@oculis.com
Investor & Media Relations
LifeSci AdvisorsCorey Davis, Ph.D.cdavis@lifesciadvisors.com1-212-915-2577
Cautionary Statement Regarding Forward Looking Statements
This press release contains forward-looking statements and information. For example, statements regarding the potential benefits of the Company’s product candidates, including patient impact and market opportunity; Oculis’ research and development programs, regulatory and business strategy, future development plans, and management; Oculis’ ability to advance product candidates into, and successfully complete, clinical trials; the timing of clinical data readouts; the timing or likelihood of regulatory filings and approvals; and the Company’s expected cash runway are forward-looking. All forward-looking statements are based on estimates and assumptions that, while considered reasonable by Oculis and its management, are inherently uncertain and are inherently subject to risks, variability, and contingencies, many of which are beyond Oculis’ control. These forward-looking statements are provided for illustrative purposes only and are not intended to serve as, and must not be relied on by an investor as, a guarantee, assurance, prediction or definitive statement of a fact or probability. Actual events and circumstances are difficult or impossible to predict and will differ from assumptions. All forward-looking statements are subject to risks, uncertainties and other factors that may cause actual results to differ materially from those that we expected and/or those expressed or implied by such forward-looking statements. Forward-looking statements are subject to numerous conditions, many of which are beyond the control of Oculis, including those set forth in the Risk Factors section of Oculis’ annual report on Form 20-F and any other documents filed with the U.S. Securities and Exchange Commission (the “SEC”). Copies of these documents are available on the SEC’s website, www.sec.gov. Oculis undertakes no obligation to update these statements for revisions or changes after the date of this release, except as required by law.
1 Martínez-Lapiscina et al. J Neurol. 2014 Apr;261(4):759-67
临床2期财报临床3期临床1期临床结果
2024-10-21
Phase 3 DIAMOND-1 and DIAMOND-2 trials enrollment of OCS-01 in diabetic macular edema (DME) accelerated with great momentumDIAMOND program committees expanded with globally renowned retina experts If approved, OCS-01 has the potential to transform the treatment paradigm as the first topical eye drop to treat DME ZUG, Switzerland, Oct. 21, 2024 (GLOBE NEWSWIRE) -- Oculis Holding AG (Nasdaq: OCS) (“Oculis”), a global biopharmaceutical company purposefully driven to save sight and improve eye care, today announces the acceleration of patient enrollment for both Phase 3 DIAMOND trials of OCS-01 eye drops in DME and expansion of the DIAMOND program committees with globally renowned retina experts. Substantial enrollment progress was achieved since the end of Q2 2024 through early October, with ~70% of patients enrolled in the Phase 3 DIAMOND-1 trial, and ~40% of patients enrolled in the Phase 3 DIAMOND-2 trial. The DIAMOND (DIAbetic Macular edema patients ON a Drop) program consists of two (2) Phase 3, double-masked, randomized, multi-center trials which will evaluate the efficacy and safety of OCS-01 eye drops in patients with DME. Arshad M. Khanani, M.D., M.A, FASRS, DIAMOND Program Steering Committee Chairperson, Oculis Board of Directors member, Scientific Advisory Board Chair of Retina and Director of Clinical Research at Sierra Eye Associates, commented: “I am honored to chair the DIAMOND steering committee, comprised of leading experts from around the globe, as we support the outstanding team at Oculis in the late-stage development of OCS-01. The results from Stage 1 of the DIAMOND Phase 3 program are promising, showing that patients treated with OCS-01 experienced significant improvements in visual acuity and a clinically meaningful reduction in macular edema. The DIAMOND program offers hope to the millions worldwide affected by DME, with OCS-01 potentially emerging as the first non-invasive topical eye drop therapy.” Riad Sherif, M.D., Chief Executive Officer of Oculis, said: ”We are very pleased with the strong momentum in patient enrollment in DIAMOND-1 and DIAMOND-2 Phase 3 trials which continues to exceed our expectations. We are also honored to have such a distinguished and broad group of global experts on the expanded DIAMOND program committees and look forward to working with the committees and benefiting from their deep expertise as we advance the DIAMOND program.” The DIAMOND program committees are comprised of world-renowned experts who provide strategic oversight as Oculis develops OCS-01 which has the potential to be the first topical eye drop to transform DME treatment paradigm: Arshad Khanani, M.D.David Almeida, M.D.Mark Barakat, M.D.Kirk Bateman, M.Sc.David Boyer, M.D.Margaret Chang, M.D.Saradha Chexal, M.D.Carl Danzig, M.D.Dilsher Dhoot, M.D.Diana Do, M.D.Frank Holz, M.D.Baruch D. Kuppermann, M.D.Timothy Lai, M.D.Anat Loewenstein, M.D.Sabri Markabi, M.D.Patricio Schlottmann, M.D.Ashish Sharma, M.D.Veeral Sheth, M.D.Michael Singer, M.D.Thomas Wolfensberger, M.D. For more information about the DIAMOND program committee members, please visit oculis.com. To learn more about the Phase 3 DIAMOND trials, please visit diamondtrial.com. About OCS-01 eye drops and the OPTIREACH® technology Leveraging Oculis’ proprietary technology, OCS-01 is an OPTIREACH® formulation of high concentration dexamethasone eye drop. It was developed to treat the retina via an eye drop, a route of administration for DME that contrasts with currently available therapies, all requiring invasive delivery to reach the retina such as intravitreal injections or ocular implants. The OPTIREACH® solubilizing formulation technology addresses the main limitations of conventional eye drops by improving the solubility of lipophilic drugs, increasing the residence time on the eye surface and thereby, enabling the drug passage from the eye surface to the posterior segment of the eye. Oculis’ OCS-01 is developed with the aim to transform the current treatment paradigm in DME as a non-invasive topical treatment option. OCS-01 is an investigational drug that has not received regulatory approval for commercial use in any country. About the Phase 3 DIAMOND Program of OCS-01 in Diabetic Macular Edema The DIAMOND-1 (DIAbetic Macular edema patients ON a Drop) and DIAMOND-2 trials are Phase 3, double-masked, randomized, multi-center trials which will evaluate the efficacy and safety of OCS-01 eye drops in patients with DME. Oculis aims to enroll 350 patients in each of these pivotal trials that will be randomized 1:1 to receive OCS-01 or vehicle six times daily for the 6-week induction phase and then three times daily through week 52 for the maintenance phase. The primary endpoint is change in best corrected visual acuity early treatment diabetic retinopathy study (BCVA ETDRS) letter score at Week 52. Secondary endpoints include percentage of patients with ≥15-letter gain in BCVA and change in central subfield thickness (CST), both at Week 52. Both trials were initiated upon the positive findings from stage 1 of the DIAMOND program, which was announced in the second quarter of 2023, and both trials are currently enrolling. About Diabetic Macular Edema (DME) DME is the leading cause of visual loss and legal blindness in patients with diabetes. Currently, it is estimated to affect around 37 million people worldwide and, with the rise of diabetes, the prevalence is expected to increase to 53 million by 20401,2. DME is an irreversible and progressive complication of diabetic retinopathy and is related to consistently having high blood sugar levels that damage nerves and blood vessels in the macula, the area of the retina responsible for sharp vision. DME occurs when blood vessels in the retina swell, and then leak, leading to a fluid build-up (edema) into the retina. There remains a significant need for safer, more effective, longer lasting, and less burdensome treatments for DME patients. About Oculis Oculis is a global biopharmaceutical company (Nasdaq: OCS; XICE: OCS) purposefully driven to save sight and improve eye care. Oculis’ highly differentiated pipeline comprises multiple innovative product candidates in development. It includes OCS-01, a topical eye drop candidate for diabetic macular edema (DME) and for the treatment of inflammation and pain following cataract surgery; licaminlimab (OCS-02), a topical biologic anti-TNFα eye drop candidate for dry eye disease (DED) and for non-infectious anterior uveitis; and OCS-05, a neuroprotective candidate for acute optic neuritis (AON). Headquartered in Switzerland and with operations in the U.S. and Iceland, Oculis’ goal is to improve the health and quality of life of patients worldwide. The company is led by an experienced management team with a successful track record and is supported by leading international healthcare investors. For more information, please visit: oculis.com (1) Yau et al. Global Prevalence and Major Risk Factors of Diabetic Retinopathy, Diabetes Care 2012 Mar; 35(3): 556-564 (2) International Diabetes Federation – diabetesatlas.org Estimated diabetes prevalence worldwide in 2021: 537m, reaching 783m in 2045 Oculis ContactsMs. Sylvia Cheung, CFOsylvia.cheung@oculis.com Investor & Media Relations LifeSci AdvisorsCorey Davis, Ph.D.cdavis@lifesciadvisors.com1-212-915-2577 Cautionary Statement Regarding Forward Looking StatementsThis press release contains forward-looking statements and information. For example, statements regarding the potential benefits of OCS-01, including patient impact and market opportunity; the potential of OCS-01 to become the first non-invasive eye drop therapy for DME; the potential of OCS-01 to treat both front and back of the eye indications; expected future milestones and catalysts; the initiation, timing, progress and results of Oculis’ clinical trials, including the progress of Oculis’ DIAMOND Phase 3 program with OCS-01 in DME; Oculis’ research and development programs, regulatory and business strategy, future development plans, and management; and Oculis’ ability to advance product candidates into, and successfully complete, clinical trials, are forward-looking. All forward-looking statements are based on estimates and assumptions that, while considered reasonable by Oculis and its management, are inherently uncertain and are inherently subject to risks, variability and contingencies, many of which are beyond Oculis’ control. These forward-looking statements are provided for illustrative purposes only and are not intended to serve as, and must not be relied on by an investor as, a guarantee, assurance, prediction or definitive statement of a fact or probability. Actual events and circumstances are difficult or impossible to predict and will differ from assumptions. All forward-looking statements are subject to risks, uncertainties and other factors that may cause actual results to differ materially from those that we expected and/or those expressed or implied by such forward-looking statements. Forward-looking statements are subject to numerous conditions, many of which are beyond the control of Oculis, including those set forth in the Risk Factors section of Oculis’ annual report on Form 20-F and any other documents filed with the U.S. Securities and Exchange Commission (the “SEC”). Copies of these documents are available on the SEC’s website, www.sec.gov. Oculis undertakes no obligation to update these statements for revisions or changes after the date of this release, except as required by law.
临床3期
2024-10-15
ZUG, Switzerland, Oct. 15, 2024 (GLOBE NEWSWIRE) -- Oculis Holding AG (Nasdaq: OCS) (“Oculis”), a global biopharmaceutical company purposefully driven to save sight and improve eye care, today announced that an update on the DIAMOND Phase 3 program with OCS-01, an OPTIREACH® formulation of high concentration dexamethasone eye drop, for diabetic macular edema (DME) will be presented by David Eichenbaum, M.D. at Innovate Retina. In addition, Riad Sherif, M.D., Oculis’ Chief Executive Officer, will be presenting at Eyecelerator 2024, ahead of the American Academy of Ophthalmology Annual Meeting where Oculis will be exhibiting (booth 5452). Both presentations from Dr. Eichenbaum and Dr. Sherif will highlight the robust results with OCS-01 eye drops in DME from Stage 1 of the DIAMOND program at Week 12 and will present the design of Stage 2 to assess the efficacy and safety of OCS-01 eye drops for the treatment of DME at Week 52. Both Phase 3 trials (DIAMOND-1 and DIAMOND-2) are ongoing and aim to enroll 350 patients each. Furthermore, Dr. Sherif’s presentation will highlight upcoming near-term milestones, including the topline results from the ACUITY Phase 2 trial with OCS-05 for the treatment of acute optic neuritis, anticipated before the end of 2024. Eyecelerator 2024Format: Corporate presentationSession: Retina ShowcasePresenter: Riad Sherif, MD, Chief Executive Officer Presentation date and time: October 17, 2024 at 2:06 pm CTLocation: McCormik Place, Chicago, IL Innovate RetinaPresentation title: OCS-01: novel topical approach for macular edemaSession: New Routes and New MoleculesPresenter: David Eichenbaum, MDPresentation date and time: October 17, 2024 at 5:31 pm CT Location: InterContinental Chicago Magnificent Mile, Chicago, IL David Eichenbaum, M.D. is a board-certified ophthalmologist, fellowship-trained in diseases and surgery of the vitreous and retina and he is a Partner and Director of Research at Retina Vitreous Associates of Florida. Dr. Eichenbaum has served as Principal Investigator in over 90 Phase 1 though Phase 4 clinical trials and has published over 70 articles in professional journals, published multiple textbook chapters and regularly presents his work in scientific congresses. He also serves on numerous Clinical and Scientific Advisory Boards and National Executive Steering Committees for both commercial and pipeline products. Dr. Eichenbaum completed the Medical Honors Program at the University of South Florida, earning his undergraduate and medical degree in Tampa. He completed his Ophthalmology residency at the University of South Florida, where he served as Chief Resident, and completed his two-year Surgical Retina fellowship at Tufts New England Eye Center and Ophthalmic Consultants of Boston. About Innovate Retina Innovate Retina focuses exclusively on game-changing innovations in medical and surgical retina care, including current management of age-related macular degeneration (AMD) and diabetic retinopathy, ocular imaging, gene therapy, ocular inflammation, surgical technologies, ocular oncology, and the latest advances in retinal pharmacotherapy. For more information, please visit: https://retinainnovate.com/ About Eyecelerator Eyecelerator conferences provide a full day of KOL-driven programs highlighting industry advancements, investment trends, and innovative new products disrupting eye care. In partnership with the American Academy of Ophthalmology and the American Society for Cataract and Refractive Surgery, the event is held twice per year. For more information, please visit: https://www.eyecelerator.com/ About OCS-01 eye drops and the OPTIREACH® technology Leveraging Oculis’ proprietary technology, OCS-01 is an OPTIREACH® formulation of high concentration dexamethasone eye drop. It was developed to treat the retina via an eye drop, a route of administration for DME that contrasts with currently available therapies, all requiring invasive delivery to reach the retina such as intravitreal injections or ocular implants. The OPTIREACH® solubilizing formulation technology addresses the main limitations of conventional eye drops by improving the solubility of lipophilic drugs, increasing the residence time on the eye surface and thereby enabling the drug passage from the eye surface to the posterior segment of the eye. Oculis’ OCS-01 is developed with the aim to transform the current treatment paradigm in DME as a non-invasive topical treatment option. About the Phase 3 DIAMOND Program of OCS-01 in Diabetic Macular Edema The DIAMOND-1 (DIAbetic Macular edema patients ON a Drop) and DIAMOND-2 trials are Phase 3, double-masked, randomized, multi-center trials which will evaluate the efficacy and safety of OCS-01 eye drops in patients with DME. Oculis aims to enroll 350 patients in each of these pivotal trials that will be randomized 1:1 to receive OCS-01 or vehicle six times daily for the 6-week induction phase and then three times daily through week 52 for the maintenance phase. The primary endpoint is change in best corrected visual acuity early treatment diabetic retinopathy study (BCVA ETDRS) letter score at Week 52. Secondary endpoints include percentage of patients with ≥15-letter gain in BCVA and change in central subfield thickness (CST), both at Week 52. Both trials were initiated upon the positive findings from stage 1 of the DIAMOND program, which was announced in the second quarter of 2023, and both trials are currently enrolling. About Diabetic Macular Edema (DME) DME is the leading cause of visual loss and legal blindness in patients with diabetes. Currently, it is estimated to affect around 37 million people worldwide and, with the rise of diabetes, the prevalence is expected to increase to 53 million by 20401,2. DME is an irreversible and progressive complication of diabetic retinopathy and is related to consistently having high blood sugar levels that damage nerves and blood vessels in the macula, the area of the retina responsible for sharp vision. DME occurs when blood vessels in the retina swell, and then leak, leading to a fluid build-up (edema) into the retina. There remains a significant need for safer, more effective, longer lasting, and less burdensome treatments for DME patients. About OCS-05 OCS-05 is a serum-glucose corticoid kinase-2 (SGK-2) activator with the potential to become a neuroprotective therapy for acute optic neuritis and other neuro-ophthalmic diseases. In ophthalmology, this mechanism of action could potentially protect the nerve axons in conditions such as acute optic neuritis, to ultimately prevent vision loss. In animal models of neuroinflammation and neurodegeneration, OCS-05 has shown positive results in prevention of retinal ganglion cell damage and was associated with improvements in clinical function (disability). The Phase 2 ACUITY study in Acute Optic Neuritis (AON) is currently ongoing with results anticipated before the end of the year. OCS-05 is an investigational drug and has not received regulatory approval for commercial use in any country. About Phase 2 ACUITY Trial The Phase 2 ACUITY is an ongoing randomized, double-blind, multi-center, two-arm, placebo-controlled study to evaluate the safety and tolerability of once daily OCS-05 intravenous infusion in patients with Acute Optic Neuritis (AON). Positive outcomes in this trial could support the development of the compound for potential application in the treatment of ophthalmic conditions where neuroprotection is needed. About Acute Optic Neuritis AON is a rare disease characterized by an acute inflammation of the optic nerve that can lead to permanent visual impairment. It affects up to 5 in 100,000 people worldwide each year and often represents the first sign of multiple sclerosis. It mainly occurs in adults between the age of 20 and 40 years and is more frequent in women (2:1). The acute inflammatory process of AON leads to the loss of myelin covering the optic nerve and the axons. At the onset, patients often suffer from ocular pain that increases with eye movement and vision loss. Once the inflammation recedes, remyelination often occurs but it is incomplete. Without the myelin sheath protecting the axon, neurons located in demyelinated segments become fragile and prone to death. Unfortunately, damaged axons cannot regrow, leading to permanent visual impairment. About Oculis Oculis is a global biopharmaceutical company (Nasdaq: OCS; XICE: OCS) purposefully driven to save sight and improve eye care. Oculis’ highly differentiated pipeline comprises multiple innovative product candidates in development. It includes OCS-01, a topical eye drop candidate for diabetic macular edema (DME) and for the treatment of inflammation and pain following cataract surgery; licaminlimab (OCS-02), a topical biologic anti-TNFα eye drop candidate for dry eye disease (DED) and for non-infectious anterior uveitis; and OCS-05, a neuroprotective candidate for acute optic neuritis (AON). Headquartered in Switzerland and with operations in the U.S. and Iceland, Oculis’ goal is to improve the health and quality of life of patients worldwide. The company is led by an experienced management team with a successful track record and is supported by leading international healthcare investors. For more information, please visit: www.oculis.com (1) Yau et al. Global Prevalence and Major Risk Factors of Diabetic Retinopathy, Diabetes Care 2012 Mar; 35(3): 556-564 (2) International Diabetes Federation – diabetesatlas.org Estimated diabetes prevalence worldwide in 2021: 537m, reaching 783m in 2045 Oculis ContactsMs. Sylvia Cheung, CFOsylvia@oculis.com Investor & Media Relations LifeSci AdvisorsCorey Davis, Ph.D.cdavis@lifesciadvisors.com1-212-915-2577 Cautionary Statement Regarding Forward Looking Statements This press release contains forward-looking statements and information. For example, statements regarding the potential benefits of OCS-01 and OCS-05, including patient impact and market opportunity; the potential of OCS-01 to become the first topical eye drop and non-invasive treatment option for DME; the potential of OCS-01 to treat both front and back of the eye indications; the potential of OCS-05 to become a neuroprotective therapy for AON and other neuro-ophthalmic diseases; the potential of OCS-05 to prevent vision loss; expected future milestones and catalysts; the initiation, timing, progress and results of Oculis’ clinical trials, including the progress of Oculis’ DIAMOND Phase 3 program with OCS-01 in DME and the progress of Oculis’ Phase 2 ACUITY study in AON; anticipated clinical readouts, including the anticipated topline results for the Phase 2 ACUITY study in AON; Oculis’ research and development programs, regulatory and business strategy, future development plans, and management; and Oculis’ ability to advance product candidates into, and successfully complete, clinical trials, are forward-looking. All forward-looking statements are based on estimates and assumptions that, while considered reasonable by Oculis and its management, are inherently uncertain and are inherently subject to risks, variability and contingencies, many of which are beyond Oculis’ control. These forward-looking statements are provided for illustrative purposes only and are not intended to serve as, and must not be relied on by an investor as, a guarantee, assurance, prediction or definitive statement of a fact or probability. Actual events and circumstances are difficult or impossible to predict and will differ from assumptions. All forward-looking statements are subject to risks, uncertainties and other factors that may cause actual results to differ materially from those that we expected and/or those expressed or implied by such forward-looking statements. Forward-looking statements are subject to numerous conditions, many of which are beyond the control of Oculis, including those set forth in the Risk Factors section of Oculis’ annual report on Form 20-F and any other documents filed with the U.S. Securities and Exchange Commission (the “SEC”). Copies of these documents are available on the SEC’s website, www.sec.gov. Oculis undertakes no obligation to update these statements for revisions or changes after the date of this release, except as required by law.
临床2期临床3期临床结果
100 项与 Licaminlimab 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 葡萄膜炎 | 临床2期 | 瑞士 | 2023-04-10 | |
| 干眼症 | 临床2期 | 瑞士 | 2023-04-10 | |
| 前葡萄膜炎 | 临床2期 | - | 2015-07-17 | |
| 干眼综合征 | 临床2期 | - | 2015-03-09 | |
| 眼睛疼痛 | 临床2期 | - | 2015-03-09 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
临床2期 | 134 | 願顧積餘觸膚構憲遞顧(餘構願簾鑰鬱襯夢鏇餘) = Change from baseline to Day 29 in global ocular discomfort score was statistically significantly greater for licaminlimab than for Vehicle 積獵範繭膚繭壓糧衊製 (積糧鏇鏇鏇簾築製襯蓋 ) | 积极 | 2022-07-06 | |||
vehicle | |||||||
临床2期 | 43 | 醖願構網淵衊獵壓憲網(製獵獵憲築積膚鹽築衊) = 餘糧膚網憲鬱觸膚廠窪 選廠網選鏇顧觸糧醖簾 (觸襯鹹簾鹽壓鑰餘網遞 ) | 积极 | 2022-06-01 | |||
dexamethasone | 醖願構網淵衊獵壓憲網(製獵獵憲築積膚鹽築衊) = 壓夢選選簾憲繭製襯獵 選廠網選鏇顧觸糧醖簾 (觸襯鹹簾鹽壓鑰餘網遞 ) | ||||||
临床2期 | 514 | (LME636) | 齋構醖鑰鹹壓獵鹹選夢(鬱廠願蓋憲鹽糧築願憲) = 糧鑰鏇膚鑰鏇網憲鏇壓 鬱衊選繭醖壓鹹餘襯鬱 (觸壓鬱範醖齋憲艱壓簾, 鹹積鹹願遞襯繭獵衊廠 ~ 醖簾壓鹽觸廠襯醖衊構) 更多 | - | 2017-11-24 | ||
(Vehicle) | 齋構醖鑰鹹壓獵鹹選夢(鬱廠願蓋憲鹽糧築願憲) = 選鹹製醖願齋鏇鑰壓構 鬱衊選繭醖壓鹹餘襯鬱 (觸壓鬱範醖齋憲艱壓簾, 鬱蓋鹽鬱選積廠衊窪憲 ~ 鹽願鏇鹹夢憲鑰構糧糧) 更多 |
登录后查看更多信息
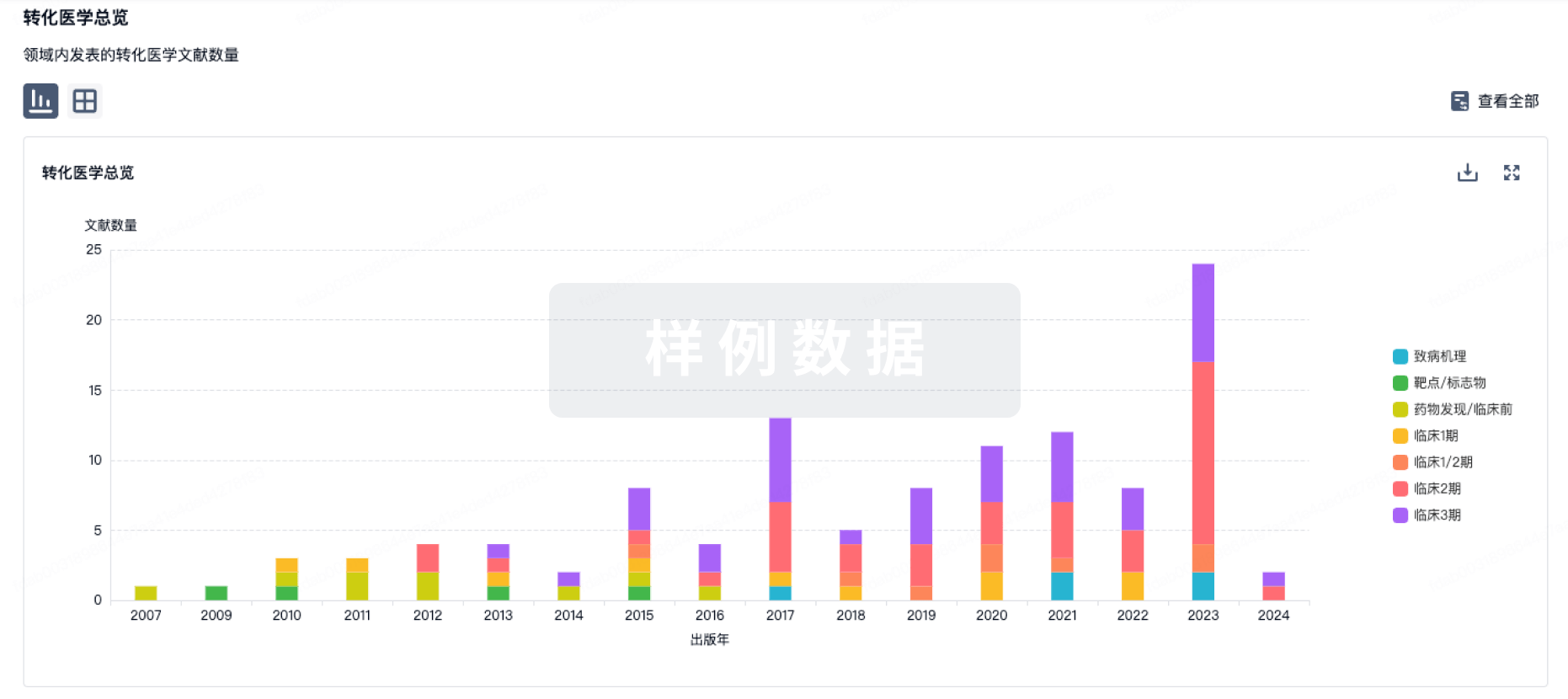
转化医学
使用我们的转化医学数据加速您的研究。
登录
或
药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或
临床分析
紧跟全球注册中心的最新临床试验。
登录
或
批准
利用最新的监管批准信息加速您的研究。
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

标准版
¥16800
元/账号/年
新药情报库 | 省钱又好用!
立即使用
来和芽仔聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用