预约演示
更新于:2025-10-14
Sonesitatug vedotin
更新于:2025-10-14
概要
基本信息
非在研机构- |
最高研发阶段临床3期 |
首次获批日期- |
最高研发阶段(中国)临床3期 |
特殊审评突破性疗法 (美国)、快速通道 (美国)、孤儿药 (美国)、突破性疗法 (中国) |
登录后查看时间轴
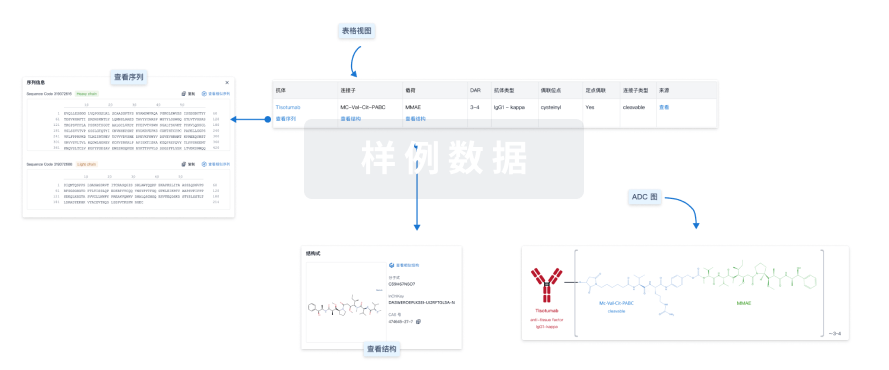
结构/序列
使用我们的ADC技术数据为新药研发加速。
登录
或
Sequence Code 1044831580H

来源: *****
Sequence Code 1044831581L

来源: *****
关联
8
项与 Sonesitatug vedotin 相关的临床试验NCT07143604
A Phase II, Multicentre, Open-label, Single-arm Study of AZD0901 Monotherapy in Second-or Later-Lines Adult Participants With Advanced/Metastatic Gastric or Gastroesophageal Junction Adenocarcinoma Expressing Claudin18.2
The purpose of this study is to measure the efficacy and safety of AZD0901 monotherapy as 2L+ treatment for participants with advanced or metastatic gastric or GEJ adenocarcinoma expressing CLDN18.2.
开始日期2025-08-29 |
申办/合作机构 |
NCT07069712
A Master Protocol of an Open-Label, Multi-Drug, Multi-Center, Phase II Platform Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Preliminary Anti-tumor Activity of Novel Agents or Combinations as Perioperative Treatment in Participants With Locally Advanced Resectable Gastroesophageal Adenocarcinoma (GEMINI-PeriOp GC)
GEMINI-PeriOp GC study will assess the safety, tolerability, pharmacokinetics (PK) and preliminary anti-tumor activity of novel agents or novel combinations as perioperative treatment in participants with locally advanced resectable gastric, gastroesophageal junction (GEJ), or esophageal adenocarcinoma who have not received previous treatment for the disease.
开始日期2025-07-17 |
申办/合作机构  AstraZeneca PLC AstraZeneca PLC [+1] |
NCT06346392
A Phase III Multi-center, Open-label, Sponsor-blinded, Randomized Study of AZD0901 Monotherapy Compared With Investigator's Choice of Therapy in Second- or Later-Line Adult Participants With Advanced/Metastatic Gastric or Gastroesophageal Junction Adenocarcinoma Expressing Claudin18.2 (CLARITY Gastric 01)
The purpose of this study is to measure the efficacy and safety of AZD0901 compared to Investigator's choice of therapy as 2L+ treatment for participants with advanced or metastatic gastric or GEJ adenocarcinoma expressing CLDN18.2.
开始日期2024-03-04 |
申办/合作机构 |
100 项与 Sonesitatug vedotin 相关的临床结果
登录后查看更多信息
100 项与 Sonesitatug vedotin 相关的转化医学
登录后查看更多信息
100 项与 Sonesitatug vedotin 相关的专利(医药)
登录后查看更多信息
4
项与 Sonesitatug vedotin 相关的文献(医药)2025-02-01·LANCET ONCOLOGY
Claudin 18.2-targeting antibody–drug conjugate CMG901 in patients with advanced gastric or gastro-oesophageal junction cancer (KYM901): a multicentre, open-label, single-arm, phase 1 trial
Article
作者: Wang, Zhen-Ning ; Shi, Xi ; Yang, Jian-Wei ; Luo, Su-Xia ; Liu, Fu-Rong ; Chen, Zhen-Dong ; Wu, Xiao-Jie ; Zhuang, Zhi-Xiang ; Ruan, Dan-Yun ; Chen, Bo ; Liang, Xiao-Hua ; Xu, Rui-Hua ; Liu, Fu-Nan ; Kumar, Rakesh ; Wei, Xiao-Li ; Zheng, Yu-Long ; Liu, Wei ; Zhang, Yan-Qiao ; Wang, Jun-Ye ; Zhang, Dong-Sheng ; Liu, Jian ; Wang, Yong-Sheng
BACKGROUND:
CMG901 is a novel first-in-class antibody-drug conjugate with a humanised anticlaudin 18.2 antibody linked to microtubule-disrupting agent monomethyl auristatin E. We aimed to assess the antitumour activity and safety of CMG901 in patients with advanced gastric or gastro-oesophageal junction cancer and other solid tumours.
METHODS:
KYM901 is a multicentre, open-label, single-arm, phase 1 trial consisting of dose-escalation and dose-expansion stages. Patients with advanced solid tumours, including gastric or gastro-oesophageal junction and pancreatic cancers, were recruited from 31 hospital sites in China. Eligible patients were aged 18 years or older, were refractory to standard therapy or had no available standard-of-care regimen, and had an Eastern Cooperative Oncology Group performance status score of 0-1, a life expectancy of at least 3 months, and at least one measurable lesion. Patients received intravenous CMG901 every 3 weeks (0·3-3·4 mg/kg in dose escalation and 2·2-3·0 mg/kg in dose expansion) until disease progression, unacceptable toxic effects, initiation of new antitumour therapy, study withdrawal, or death. Primary endpoints were adverse events and dose-limiting toxic effects in the dose-escalation phase, and objective response rate and recommended phase 2 dose in the dose-expansion phase. Confirmed objective response was defined as a partial or complete response that was verified by follow-up imaging at least 4 weeks after the initial assessment. Safety was assessed in all patients who received at least one dose of CMG901 with at least one post-dose safety evaluation. Antitumour activity was assessed in all patients who received at least one dose of CMG901 (full analysis set) and in all CMG901-treated patients with at least one post-dose imaging evaluation and no major protocol deviations (efficacy analysis set). Dose-expansion data for patients with pancreatic cancer will be published separately. Due to small sample sizes, results in patients with other solid tumours (n=2) are not planned for publication. This ongoing trial is registered with ClinicalTrials.gov, NCT04805307.
FINDINGS:
Between Dec 24, 2020, and Feb 23, 2023, 27 patients were enrolled in the dose-escalation phase (median age 57·0 years [IQR 48·0-63·0]; 14 [52%] male, 13 [48%] female) and 107 patients with gastric or gastro-oesophageal junction cancer in the dose-expansion phase (median age 56·0 years [44·0-64·0]; 57 [53%] male, 50 [47%] female). As of Feb 24, 2024, one dose-limiting toxic effect (grade 3 pancreatitis) occurred at 2·2 mg/kg, and the maximum tolerated dose was not reached in the dose-escalation phase. All 27 patients reported at least one treatment-emergent adverse event, most frequently vomiting (19 [70%]), decreased appetite (16 [59%]), proteinuria (16 [59%]), and anaemia (15 [56%]), and five (19%) had drug-related grade 3 or worse treatment-emergent adverse events. In 107 patients, grade 3 or worse treatment-emergent adverse events occurred in 73 (68%) patients and serious adverse events occurred in 54 (50%) patients in dose expansion. The most common grade 3-4 adverse events were neutrophil count decreased (22 [21%]), anaemia (15 [14%]), and vomiting (11 [10%]). One treatment-related death was reported. At median follow-up of 9·0 months (IQR 4·4-12·9), among 113 patients with gastric or gastro-oesophageal junction cancer in the 2·2-3·0 mg/kg cohort full analysis set across both the dose-escalation and dose-expansion phases, the confirmed objective response rate was 28% (95% CI 20-38; 32 of 113 patients). In the 109 patients included in the efficacy analysis set, the confirmed objective response rate was 29% (95% CI 21-39; 32 of 109 patients). Based on overall safety, activity, and pharmacokinetics of CMG901, 2·2 mg/kg was the proposed recommended phase 2 dose.
INTERPRETATION:
CMG901 showed a manageable safety profile and had promising antitumour activity in patients with advanced gastric or gastro-oesophageal junction cancer.
FUNDING:
KYM Biosciences.
2024-09-01·Cell Reports Medicine
CMG901, a Claudin18.2-specific antibody-drug conjugate, for the treatment of solid tumors
Article
作者: Wang, Ying ; Chen, Bo ; Zhang, Libo ; Xu, Ruihua ; He, Yanyun ; Liu, Wei ; Song, Qin ; Wei, Xiaoli ; Li, Zhiyao ; Liu, Furong ; Xu, Gang ; Wang, Changyu
Claudin18.2 has been recently recognized as a potential therapeutic target for gastric/gastroesophageal junction or pancreatic cancer. Here, we develop a Claudin18.2-directed antibody-drug conjugate (ADC), CMG901, with a potent microtubule-targeting agent MMAE (monomethyl auristatin E) and evaluate its preclinical profiles. In vitro studies show that CMG901 binds specifically to Claudin18.2 on the cell surface and kills tumor cells through direct cytotoxicity, antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and bystander killing activity. In vivo pharmacological studies show significant antitumor activity in patient-derived xenograft (PDX) models. Toxicity studies show that the major adverse effects related to CMG901 are reversible hematopoietic changes attributed to MMAE. The highest non-severely toxic dose (HNSTD) is 6 mg/kg in cynomolgus monkeys and 10 mg/kg in rats once every 3 weeks. CMG901's favorable preclinical profile supports its entry into the human clinical study. CMG901 is currently under phase 3 investigation in patients with advanced gastric/gastroesophageal junction adenocarcinoma expressing Claudin18.2 (NCT06346392).
2024-01-12·Cancer discovery
Optimism Surrounds Claudin 18.2 ADC
Article
Abstract:
In a phase I trial of CMG901—a first-in-class antibody–drug conjugate targeting claudin 18.2—researchers found that it can best current therapies for treating gastric and gastroesophageal cancers. Historically, progression-free survival (PFS) hovers around 7 months and overall survival (OS) around 14 to 16 months. However, depending on the dose, PFS with CMG901 ranged from 3.3 to 14.5 months, and OS ranged from 8.5 months to not reached, after a median follow up of 6 months.
286
项与 Sonesitatug vedotin 相关的新闻(医药)2025-10-12
随着国内医药创新的政策生态环境不断完善,创新药研发迎来快速发展,全球竞争力显著提升。笔者从药物管线存量、创新药管线增量、头部企业竞争等维度,分析总结近年中国医药创新在全球竞争维度上取得的进步与仍待补足之处。
规模:稳居全球第二
截至2025年1月,中国在研药物数量达到7041个,同比增长15.1%。我国管线规模仅次于美国,稳居全球第二位,全球占比升至29.5%(详见图1)。
实际上,中国管线扩张速度高于全球。2015年以来,中国在研药物数量始终保持了两位数的增长,2019年增幅达到40%的峰值后有所回落,但始终高于全球管线的扩张速度(详见图2)。2016-2025年,中国药物管线十年复合增长率为23.5%,较全球同期(6.9%)高近17个百分点。
中国管线全球占比也在持续上升。随着在研药物数量快速增加,中国管线规模在全球的占比也不断提升。2015年,中国管线的全球占比仅为6.9%,2019年升至两位数(12.6%),2022年突破两成(20.9%),到2025年已近三成(29.5%)。与2015年相比,中国管线的全球占比提升了22.6个百分点。
中美管线规模差距显著缩小。美国是全球药物研发最大的动力来源,但近年来管线扩张速度显著减缓。与此同时,中国管线规模快速增长,与美国的差距已显著缩小(详见图3),2015年,美国和中国在研药物数量分别为7034个、851个,美国管线规模约是中国的8倍;而到2025年,美国与中国的在研药物数量分别为11455个、7041个,美国管线规模为中国的1.6倍。
增量:创新力量涌动
在创新药管线方面,中国所取得的进步更为亮眼。2015—2024年,中国企业研发的活跃状态创新药数量累计为3575个,已高于美国(2967个),位居全球首位(详见图4)。中国的创新药研发管线中,细胞疗法与小分子类创新药最多,分别占比28%和19%,其他新技术产品如双/多抗类药物、放射性药物、抗体偶联药物(ADC)、基因疗法也逐渐增多。目前大多数创新药仍处于Ⅰ期试验,占比达58%。
中国每年新进入临床的创新药数量迅速增长。2015年,中国首次开展临床试验的原研创新药数量为124款,低于美国(283款)与欧洲(199款)(详见图5)。2020年起,中国企业每年首次开展临床试验的原研创新药数量开始超过美国,2024年达到704款。当前,美国企业研发的创新药数量每年为400~500款,欧洲每年约有200款创新药进入临床,日本则在100款以下。
全球首次开展临床试验的创新药的中国贡献份额也在持续提升。2015年,中国企业研发的创新药数量在全球占比为17.3%,较同期美国(39.5%)低20多个百分点(详见图6)。此后,中国对全球创新药的贡献比重稳步上升,美国与欧洲的比重有所下滑。2024年,全球首次开展临床试验的创新药中近半来自中国(45%)。
与此同时,中国企业的研发覆盖度与进度有待进一步提升。目前,全球在研创新药共涉及3212个赛道(根据靶点和药品类型对创新药进行归类,具有相同靶点和药品类型的创新药被视为同一个赛道)。中国企业在研产品覆盖了其中1300个赛道,覆盖度为40.5%。而美国企业的在研产品覆盖了1689个赛道,覆盖度较中国高出12.1%。此外,研发进度上中国也存在显著差距。中国产品在716个赛道(22.3%)上研发进度排名第一,而美国在其中1212个(37.7%)赛道的研发进度排名第一。
趋势:同类首创崛起
在最能体现创新成色的“First-in-Class(FIC,同类首创)”药物领域,中国企业也取得了明显进步。长期以来,中国药品研发主要以同类药物(Me-too)和改进型药物(Me-better)为主,在临床价值政策导向的持续推动下,越来越多的中国企业逐步转向创新性更强的源头创新,在全球竞争中从快速跟随(Fast-follow)迈向同类最优(Best-in-Class,BIC)和FIC迈进。
2015年,中国企业进入临床的FIC创新药只有9个,在全球的占比不足10%;2021年,中国进入临床的FIC创新药数量首次超过欧洲,位居全球第二;2024年,这一数字增加至120个,在全球的占比超过30%(详见图7)。
从药物类型来看,2015—2024年,中国企业原研的FIC创新药中,细胞疗法最多,其次为小分子、放射性药物和双/多抗,超过90%的药物尚处于临床早期阶段。在一些全球热门研发领域,中国企业已具备显著竞争力。例如,康诺亚与美雅珂开发的CMG901,是全球进度最快的靶向CLDN18.2的ADC药物,目前已进入胃癌的Ⅲ期临床试验;亘喜生物开发的GC012F,是全球进度最快的CD19/BCMA双靶点CAR-T细胞疗法。
但需指出的是,虽然中国在FIC药物管线方面取得了显著进步,管线规模仍与美国存在较大差距,且大部分管线仍处于早期阶段,离上市尚远。2015—2024 年,中国企业研发的活跃状态FIC创新药数量累计为629个,在全球占比23.6%;美国这一数值达到1152个,全球占比超过四成。
在FIC药物上市方面,目前国内上市的FIC药物主要由跨国药企巨头主导。2015—2024年,在中国获批上市的FIC创新药累计有153款,其中超过90%为进口产品,国产FIC创新药产品仅有11款。
2015—2024年,全球平均每年有27款FIC创新药获批上市,累计数量达268款。其中,在美国首发的FIC创新药占64.6%,欧洲和日本的占比分别为16%和13.1%,而首发在中国的FIC创新药占比较小(详见图8)。
份额:占比持续扩大
截至2025年1月,全球医药研发企业中有17%将总部设在中国(1181家),虽然与美国39%的占比仍存在较大差距,但明显领先于其他国家。
中国作为全球医药研发热土的地位持续提升。2016年,总部设在中国的医药研发企业数量在全球的占比仅为4%;2017年追上加拿大,2019年超过英国,此后占比保持上升态势,稳居全球第二,与美国之间的差距也在缩小(详见图9)。
链接<<<
头部企业创新能力稳步提升
近两年,跻身全球管线规模Top25企业之列的中国药企数量稳定在4家。自2020年首次有中国药企跻身全球管线规模Top25企业以来,中国力量在全球研发头部行列中稳定扩容。
2020年,李氏大药厂以第21的排位,成为首家跻身全球管线规模Top25的中国药企;虽然李氏大药厂次年即跌出名单,但恒瑞医药、复星医药两家企业在2022年携手登榜,分列第16位、第23位;2023年,石药集团首次入榜全球管线规模Top25企业,使得跻身全球药物研发头部行列的中国企业数量达到3家;2024年,中国生物制药管线迅速扩容,全球排名升至第15位,管线规模Top 25的中国企业数量增至4家,且恒瑞医药排名持续上升,成为首个进入全球管线规模十强的中国药企;2025年,百济神州首进榜,填补复星医药的空缺,进入全球管线规模Top25的中国企业仍为4家(详见表1)。
中国头部企业仍需继续追赶全球前排。对比中美两国管线规模前十企业,差距依旧十分显著。从全球排位看,2025年美国管线规模前十企业中,仅再生元与渤健未进入全球管线Top25药企之列,而国内上榜企业只有4家,其中百济神州为首次上榜。
从管线规模看,管线超过200个药物的美国企业有5家,超过100个药物的美国企业有8家(详见表2)。其中,辉瑞在研药物数量达到271个,位居全球第一。中国尚无超过200个管线的企业,超过100个药物的有恒瑞医药、中国生物制药、石药集团3家,其中恒瑞医药以173个在研药物领衔国内企业。(晴雪)
编辑:张洁莹
版式编辑:于成林
审校:马飞、张松
30亿畅销药攻防战!晖致首仿,华东突袭,AZ还能守住霸主地位?
事关中药注射剂上市后研究和评价工作,公开征求公众意见
31省(区、市)建医保电子处方中心,处方外流全面推进
www.yyjjb.com.cn
洞悉行业趋势
长按关注医药经济报
《中国处方药》
学术公众号
聚焦药学学术和循证研究
长按关注中国处方药
《医药经济报》
终端公众号
记录药品终端产经大事件
长按关注21世纪药店
抗体药物偶联物基因疗法放射疗法临床研究细胞疗法
2025-09-24
·小药说药
-01-
引言
胃癌在癌症相关死亡率中排名第三,被认为是全世界最难治愈的癌症之一。在晚期或转移性胃癌或胃食管交界处(GEJ)腺癌患者中,中位总生存期(mOS)不超过10个月。虽然人类表皮生长因子受体2(HER-2)靶向治疗和免疫检查点抑制剂已经为特定人群带来福音,但在进展期胃癌中寻找其他靶点势在必行,claudin18.2(CLDN18.2)随之而来。
Claudins是一个蛋白质家族,其作用是维持控制细胞间分子交换的紧密连接。广泛分布于胃、胰腺和肺组织,可用于诊断和治疗。CLDN18.2亚型是一种胃特异性亚型,自从Sahin发现CLDN18.2是一种高度选择性的分子,并且只在癌细胞中广泛表达,它就成为一种理想的靶点。CLDN18.2通常埋藏在胃粘膜中,正常组织中的单克隆抗体基本上接触不到,恶性肿瘤的发生会导致紧密连接的破坏,使肿瘤细胞表面的CLDN18.2表位暴露出来,成为特定的靶点。因此,CLDN18.2赋予靶向治疗的特异性。最近发现在胰腺癌(50%)、食管癌和肺癌中的表达也显示了诊断和治疗其他肿瘤的潜力。
-02-
一、Claudin18.2的结构和生物学作用
Claudins是一个组成紧密连接(TJs)的完整膜蛋白家族,TJs是主要的细胞间连接,充当通透性屏障,通过划分膜上下区域赋予上皮细胞极性。目前,哺乳动物claudin家族由27种蛋白质组成,许多选择性剪接claudin蛋白在各种组织中表达。
Claudins是四次跨膜蛋白,包括四个跨膜结构域(TM1-4)、细胞内N端和C末端以及两个细胞外环(ECL1和ECL2)。ECL1包含四条β链和一条胞外螺旋(ECH),ECL2包含一条β链和一个细胞表面暴露的部分跨膜结构域。
ECL参与claudin链之间相互作用的形成,并通过两个可变区域形成基于claudin的闸门功能。根据生理学研究,提出了两种闸门功能机制:“孔隙”机制,其中溶质或离子通过由TJ链形成的细胞旁通道,以及“泄漏”机制,溶质可能通过TJ链中的断裂。此外,通过与许多其他信号分子的相互作用,claudin主要作为抑制因子发挥作用,从而影响细胞生长、存活、增殖和分化。
-03-
二、Claudin18.2异常的致癌作用
包括claudins在内的紧密连接复合体被认为在致癌作用中具有抑制作用,类似于细胞粘附分子的肿瘤抑制功能。CDH1突变引起的钙粘蛋白表达不足已认为是肿瘤发生的机制之一。大约20-30%的胃癌claudin 18.2表达下调或缺失,claudin 18.2的下调也被认为与癌细胞侵袭和增殖水平的增加有关。然而,支持claudin 18.2下调与胃癌发生之间相关性的证据仍然有限。
除了claudin 18.2表达的变化外,在胃癌患者中还检测到涉及CLDN18的融合。在3%的胃癌患者和10%的扩散型胃癌患者中检测到CLDN18-ARHGAP融合基因。这些融合破坏了连接复合体,可能是由于C末端结构域的缺失以及由此导致的与肌动蛋白调节蛋白的相互作用的缺失,再加上ARHGAP的Rho-GTPase结构域的异位定位,这种破坏导致转化蛋白RHOA的下游失活,可能导致恶性转化。然而,目前仍然没有确凿的证据表明claudin 18.2的表达与致癌作用之间存在直接联系。
-04-
三、Claudin18.2阳性胃癌的临床病理特征
各种研究已经描述了claudin 18.2在胃癌中表达的临床病理特征和预后意义。在SPOTLIGHT和GLOW试验中使用了43-14A抗claudin 18抗体识别claudin 18.1和claudin 18.2的C末端。使用43-14A分析的样本的阳性标准被定义为IHC评分>2+和染色≥癌细胞表面积的75%。
在SPOTLIGHT和GLOW试验的联合分析中,38.4%的患者被认为患有claudin 18.2阳性肿瘤。当排除HER2阳性癌症患者时,这一百分比增加到42.3%。白人(42.3%)和亚裔(36.4%)患者的claudin 18.2阳性率存在统计学显著差异(P = 0.0004)。此外,根据对SPOTLIGHT和GLOW试验样本的生物标志物分析,中国大陆claudin 18.2阳性胃癌患者的百分比(35.0%)与北美(37.7%)相似,但低于欧洲和中东(44.0%)。claudin 18.2的表达与年龄或性别之间也存在统计学上显著的相关性。在≤65岁的患者(40.9%)中观察到的claudin 18.2阳性肿瘤比例高于>65岁的患者(34.3%),在≤75岁的患者中(39.0%)高于>75岁的患者(30.9%)。claudin 18.2阳性癌症的女性患者比例(43.7%)高于男性患者(36.9%)。在弥漫型组织学肿瘤中观察到claudin 18.2阳性的发生率(48.3%)高于肠型组织学(38.8%)。
值得注意的是,尽管已经报道了claudin 18.2状态与几个患者或临床特征之间具有统计学意义的相关性,但大多数组之间claudin 182阳性率的数字差异通常很小。此外,胃癌和GEJ癌之间claudin 18.2阳性率没有一致性差异。
-05-
四、Claudin18.2阳性胃癌的基因组和免疫学特征
分子表征方法的进展揭示了胃癌独特的分子特征。在TCGA分析中,研究人员提出了四种分子亚型:EB病毒(EBV)阳性、微卫星不稳定(MSI)、基因组稳定(GS)和染色体不稳定(CIN)肿瘤。一项研究的结果表明,Claudin18.2在EBV阳性癌症中的阳性率仅为26%。关于claudin 18.2在其他TCGA癌症亚型中表达率的数据有限,claudin 18.2在20.8%的错配修复缺陷(dMMR)癌症中表达。其他研究报道了claudin 18.2阳性与MSI-H之间的有限关联,特别是对于具有高水平claudin 18.2-表达的肿瘤。
claudin 18.2的表达与其他生物标志物之间的关系也有研究。三项独立的回顾性研究分析了claudin 18.2和HER2的重叠表达,患者的重叠阳性率约为3.4%-4.9%。对SPOTLIGHT和GLOW数据的探索性分析表明,17.2%的claudin 18.2阳性进展期胃癌(AGCs)也是PD-L1阳性(定义为CPS≥5)。
此外,还评估了claudin 18.2阳性胃癌及其肿瘤微环境(TME)的免疫学特征。自然杀伤(NK)细胞计数减少和中性粒细胞计数升高是claudin 18.2阳性胃癌的普遍特征。转录分析还表明,TME中调节性CD4+T细胞、CD8+T细胞和髓系树突状细胞数量有限,B细胞水平升高,而巨噬细胞存在的数据在各研究中不一致。对80个癌症标本的多重IHC分析显示,在claudin 18.2阳性肿瘤中,不表达免疫检查点和/或其相关配体(如PD-1、PD-L1、LAG3和TIM3)的CD8+T细胞比例较高。这些发现表明,claudin 18.2阳性胃癌可能具有“冷”免疫表型,具有有限的免疫细胞浸润和免疫抑制性TME。
-06-
五、Claudin18.2的靶向治疗
Claudin 18.2作为极具前景的肿瘤靶向治疗靶点,众多创新药企争相布局,成为必争之靶点高地。目前为止,国际国内20多家药企布局了claudin 18.2靶向药物的临床开发,大部分在研项目属于单靶点抗体药物,此外还包括claudin 18.2双抗药物、ADC和CAR-T等。
单克隆抗体
Zolbetuximab(IMAB362,claudixmab)是第一种针对该靶点的开发药物,是一种嵌合的IgG1单克隆抗体,在肿瘤细胞表面与CLDN18.2特异结合,从而引发抗体依赖性细胞毒性(ADCC)、补体依赖性细胞毒性(CDC),凋亡和抑制细胞增殖。临床前研究已经成功地证实了它具有清除癌细胞和控制疾病的强大能力。随后,通过多个I/II期试验评估了其临床疗效和安全性。
第一项人体临床试验(NCT00909025)旨在确定剂量递增队列中Zolbetuximab的最大耐受剂量和推荐剂量,15名先前接受过治疗的进展期胃腺癌/GEJ腺癌患者进入评估。最后评估使用600 mg/m2的剂量为后续的推荐研究剂量。
IIa期临床试验(NCT01197885,MONO 2013)研究了其对54例难治性晚期或转移性CLDN18.2阳性胃腺癌患者的疗效和安全性。研究结果显示,中位无进展生存期(mPFS)提高到14.5周,受试者的平均响应时间延长至24.6周。10例患者获得临床改善,其中PR 4例(9%),SD 6例(14%),其中90%的患者CLDN18.2高表达。
2023年7月6日,根据SPOTLIGHT和GLOW的数据,美国食品药品监督管理局(FDA)授予Zolbetuximab优先审评地位,作为不可切除、局部晚期或转移、HER2阴性、claudin 18.2阳性胃癌或GEJ腺癌患者的一线治疗。此外,其它进入临床研究的单克隆抗体还包括Osemitamab(TST001)、AB011、MIL93、ASKB589和ZL-1211等。
双特异性抗体
Gresonitamab、ASP2138、AZD5863和QLS31905都是设计用于结合claudin 18.2和CD3的双特异性抗体。Gresonitamab已在一项涉及AGC或PDAC患者的国际多中心I期研究中进行了测试(NCT04260191)。QLS31905在一项I期试验中进行了测试,该试验涉及经过大量预处理的晚期实体瘤患者,在接受剂量≥200μg/kg的27名患者中,ORR和DCR分别为11.1%和63.0%。在胃癌或GEJ癌患者中未观察到任何反应。测试ASP2138(NCT05365581)和AZD5863(NCT06005493)的I期研究正在进行中。
Q-1802是一种靶向claudin 18.2和PD-L1的人源化双特异性抗体。Q-1802正在一项开放标签单臂I期试验中进行测试(NCT04856150)。剂量递增阶段已经完成,未检测到剂量限制毒性,最高可达20 mg/kg剂量。恶心(62.1%)、呕吐(62.1%)和腹痛(27.6%)是最常见的任何级别的不良事件。在剂量扩展队列的9名患者中,2名患者出现部分响应,4名患者疾病稳定。
PT886是一种针对claudin 18.2和CD47的新型双特异性抗体。该药物正在一项开放标签的I期试验中进行测试,该试验涉及晚期胃癌和GEJ腺癌或PDAC患者。
Givastomig(TJ-CD4B/ABL111)是一种创新的双特异性抗体,同时靶向claudin 18.2和4-1BB。Givastomig目前正在美国和中国的中心进行一期试验(NCT04900818)。这项研究的初步数据表明,有三名患者(16.6%)出现部分反应。常见的毒性包括1-2级恶心(22%)、疲劳(14%)和呕吐(12%),共报告了7起3级事件。
CAR-T细胞
CT041是靶向claudin 18.2的第二代CAR-T细胞产品,包含CD8α铰链区、CD28共刺激结构域和CD3ζ信号结构域。临床前数据表明,在小鼠异种移植物模型中,具有强大的抗肿瘤活性,不会对非恶性胃组织或其他器官造成明显损伤。在一项I期研究中,CT041在转移性claudin 18.2阳性的胃腺癌或胰腺癌患者中显示出相当大的潜力(ORR 33.3%,中位PFS 130天)。在第二阶段试验中,所有癌症患者的ORR为48.6%,胃癌为57.1%;DCR所有癌症为73.0%,胃癌为75.0%。
其他几种claudin 18.2靶向CAR T细胞疗法目前正在早期研究中进行测试,包括配备有4-1BB共刺激结构域的第二代CAR LB1908以及第四代CAR-T,第四代的两种细胞都被设计为分泌特异性细胞因子和趋化因子RD07(NCT05284968)和CT048(NCT05393986)。
ADC
SYSA1801(EO3021)是一种特异性靶向claudin 18.2的ADC,其使用药物抗体比(DAR)约为2的MMAE有效载荷。在一项正在进行的I期试验中,33名晚期、重度预处理的claudin 18.2阳性胃癌或胰腺癌患者接受了SYSA1801单药治疗。在21名可评估疗效的患者中,观察到ORR为38.1%,DCR为57.1%,AGC患者的ORR和DCR分别增加到47.1%和64.7%。大多数接受的患者出现恶心和呕吐,≥3级的反应为42.9%和28.6%。33名患者中有7名(21.2%)出现干眼症,这也被认为与治疗有关。
CMG901(AZD0901)是以一种人源化IgG1抗claudin 18.2抗体,该抗体通过DAR约为4的可裂解连接子与MMAE有效载荷偶联。CMG901显示出强大的临床前活性。在包含113名晚期、预处理的GEJ或胃癌患者的I期临床试验中,ORR为42%,最常见的不良反应为贫血和中性粒细胞计数减少。
此外,其他大多数ADC仍处于早期临床测试中,包括TPX-4589/LM-302(NCT05001516、NCT05934331)、RC118(NCT04914117、NCT05205850)、SKB315(NCT05367635)、SOT102(NCT05525286)、TORL-2-307-ADC(NCT05156866)和JS107(NCT05502393)。
其它疗法
BNT141是一种创新的基于RNA的治疗药物,编码包裹在脂质纳米颗粒中的抗claudin 18.2抗体,是该类治疗药物中首批进入临床测试的产品之一。BNT141的临床前评估表明,与传统抗体药物相比,剂量-暴露比更有利。一项测试BNT141的I/II期、开放标签、多中心试验(NCT04683939)于2020年12月启动,但申办方于2023年10月停止了这项研究,原因未披露。
-07-
四、CLDN18.2靶向治疗的未来
首先,CLDN18.2可能是继HER-2之后胃癌的第二重要靶点。在高表达的人群,它甚至超过了HER-2。FAST试验中OS的疗效不亚于HER-2。需要进一步研究以确定CLDN18.2水平的理想界限值,以获得最佳效益。
第二,联合治疗是值得期待的。随着Zolbetuximab联合化疗方案的兴起,与其他靶向药物联合治疗可能也值得研究。值得注意的是,联合应用Zolbetuximab和免疫治疗可刺激T细胞浸润,这与免疫检查点抑制剂相协调。
最后,更多的预测预后的意义需要进一步的调查。分子亚型在指导精确的药物治疗方面具有更大的潜力,如CLDN18-ARHGAP26/6融合,表明生存率和化疗抵抗力较差。更多的CLDN18.2的预测因子可以被探索和验证,从而使特殊人群受益。
CLDN18.2在HER-2阴性胃癌患者中是一个很有前途的靶点,不仅选择性高,而且在人群患病率方面也很有潜力,是胃癌靶向治疗的理想补充。新型抗体zolbtuximab,无论是单药治疗还是联合化疗,都显示出显著的优越疗效和安全性。CLDN18.2CAR-T细胞治疗最近以其优异的表现探索了另一种可能性。该策略将进展到第三阶段临床试验,当与其他疗法相结合时,将可能进一步的提升疗效。随着分子结构和临床试验的不断完善,抗CLDN18.2治疗将完美地回答各种疑问,并在未来带来更多惊喜。
参考文献:
1. Claudin 18.2 as a novel therapeutic target. Nat Rev Clin Oncol. 2024 Mar 19
2. Evaluation and reflection on claudin 18.2 targeting therapy in advanced gastric cancer. Chin J Cancer Res. 2020 Apr; 32(2): 263–270.
3. clinicaltrials.gov
“小药说药知识文库”已落户知识星球,持续更新最新生物医药、免疫学、肿瘤学相关知识内容,加入可免费下载最新内容的精选PPT,加入方法在知识星球APP搜索“小药说药知识文库”或扫描下方二维码,小药邀请你到知识星球一起学习!
公众号已建立“小药说药专业交流群”微信行业交流群以及读者交流群,扫描下方小编二维码加入,入行业群请主动告知姓名、工作单位和职务。
免疫疗法
2025-09-22
·抗体圈
摘要:紧密连接蛋白亚型 Claudin18.2(CLDN18.2)仅在正常胃黏膜上皮细胞中表达,调控物质跨膜运输。但在正常组织的恶性转化中 CLDN18.2 得以保留,在胃癌、胰腺癌、乳腺癌等多种恶性肿瘤中普遍表达。以 CLDN18.2 为靶点的抗体偶联药物(antibody-drug conjugates,ADC)同时具有单克隆抗体的高靶向选择性和细胞毒性药物的高抗肿瘤活性,是极具治疗前景的新型生物靶向抗肿瘤药物。本文对 CLDN18.2 ADC 的临床试验进展进行综述,为相关药物的研发提供一定参考。
抗体偶联药物(antibody-drug conjugates,ADC)是由连接子偶联单克隆抗体、小分子细胞毒药物形成的一类新型靶向抗肿瘤药物。理想的 ADC 药物在血液循环中保持稳定、无或低毒性,当 ADC 的单克隆抗体组件与肿瘤细胞特异性高表达的靶抗原特异性结合时,ADC 即被肿瘤细胞内吞形成内体,经溶酶体作用降解释出所负载的细胞毒性药物从而杀伤肿瘤细胞。除了细胞毒性药物的直接抗肿瘤活性,ADC 还可通过抗体依赖性细胞毒性作用(antibody-dependent cellular cytotoxicity,ADCC)、抗体依赖性细胞吞噬作用(antibody-dependent cellular phagocytosis,ADCP)和补体依赖性细胞毒性作用(complement-dependent cytotoxicity,CDC)诱导肿瘤细胞凋亡。部分具有膜渗透性的细胞毒性药物还能通过旁观者效应扩散至靶细胞的邻近细胞,从而诱导抗原表达阴性的肿瘤细胞凋亡。ADC 同时具有化疗药物对肿瘤细胞的强杀伤作用与靶向治疗的精准性、低全身毒性,是一类强大的靶向抗肿瘤药物。
紧密连接蛋白 Claudins(CLDNs)是一类调控邻近细胞间离子、溶质等物质流通的膜蛋白,其亚型 Claudin18.2(CLDN18.2)在胃癌、胰腺癌、结直肠癌、肺癌、乳腺癌等多种恶性肿瘤组织中均被检出特异性表达。同时,增殖速度快、易侵袭转移的肿瘤细胞间连接更为松散,其膜表面的 CLDN18.2 较正常细胞更易暴露而被单抗识别。这些特性使 CLDN18.2 成为恶性实体瘤免疫治疗的 “黑马” 靶点,以之为靶点研发的抗体偶联药物将是靶向及免疫治疗获得性耐药患者的又一理想治疗药物,其作用机制见图 1。
图1 CLDN18.2ADC作用机制
随着 IBI343、CMG901(AZD0901)于 2024 年 2 月先后注册正式成为全球首批进入 Ⅲ 期临床试验的在研 CLDN18.2 ADC 新药,TPX-4589(LM-302)紧随其后成为第 3 个准行 Ⅲ 期临床试验的 CLDN18.2 ADC,又有 SHR-A1904、BL-M05D1 等在近年陆续进入临床试验。目前国内外已有超 10 款临床在研的 CLDN18.2 ADC 新药(见表 1)。
表 1 临床在研 CLDN18.2 ADC 详情
(注:“-” 表示暂未披露药物详细信息)
本文旨在对 Claudin18.2 抗体偶联药物的研发应用现状进行梳理,以期更好地帮助科研工作者和临床医生研发并应用 CLDN18.2 ADC。1 负载 DNA 拓扑异构酶抑制剂的 CLDN18.2 ADC
DNA 拓扑异构酶能够调节真核细胞 DNA 的拓扑结构,维持 DNA 复制和转录活动的正常进行。DNA 拓扑异构酶抑制剂则可通过破坏 DNA、拓扑酶结构,或形成 DNA - 拓扑酶 - 抑制剂三元复合物,干扰 DNA 的复制和转录,从而抑制肿瘤细胞增殖,是目前 CLDN18.2 ADC 的常用载荷之一。1.1 BL-M05D1
BLM05D1 是百利药业公司研发的一种 CLDN18.2 ADC,其有效负载 Ed-04 是生物碱喜树碱的衍生物,能够抑制 DNA 拓扑异构酶 Ⅰ,阻止 DNA 和 RNA 的合成并诱导 DNA 损伤,阻断细胞周期于 S 期,从而诱导细胞凋亡,实现抗肿瘤作用。同时 BLM05D1 应用一种针对 CLDN18.2 的新型特异性单抗 5103F3,能够介导 ADCC、CDC 间接杀伤肿瘤细胞;高度稳定的组织蛋白酶 B 可裂解接头与高达 8 的药物抗体比(drug-to-antibody ratio,DAR,即平均每个抗体携带 8 个有效载荷分子),有效提高了药物的选择毒性。基于各组件的共同作用,在临床前研究中 BLM05D1 对人胃癌、胰腺癌 PDX 模型均表现出较强的肿瘤抑制能力,为进一步评估其疗效与安全性,百利药业公司申请了 BL-M05D1 在局部晚期或转移性实体瘤患者中的 Ⅰ 期临床研究(CTR20241139)。1.2 IBI343
创新型 CLDN18.2 ADC——IBI343 可从两方面实现抗肿瘤活性:①负载 DNA 拓扑异构酶 Ⅰ 抑制剂 Exatecan,直接干扰肿瘤细胞的 DNA 合成和复制,抑制其增殖。②被递送至靶细胞释出细胞毒性药物后通过旁观者效应杀伤邻近的 CLDN18.2 阴性的肿瘤细胞。此外,IBI343 在体内、外与包括免疫检查点阻滞剂、DNA 损伤修复途径抑制剂在内的各抗癌药物均显示强协同作用。同时 IBI343 还显示良好的安全性,在恒河猴药物非临床研究质量管理规范(Good Laboratory Practice,GLP)药物毒理学实验中得到 IBI343 的最高非严重毒性反应剂量为 30mg・kg⁻¹,主要可见靶点药理学作用相关的呕吐、白蛋白(albumin,ALB)降低、胃黏膜糜烂、萎缩等不良反应,并且在该剂量下毒副作用具有可逆转性,停药恢复 6 周后实验动物各指标均已正常。
近期信达生物公司公布了 IBI343 对晚期胰腺导管腺癌或胆道癌患者的部分临床试验数据(NCT05458219):截至 2024 年 1 月 15 日,患者用药后客观缓解率(objective response rate,ORR)为 32.3%(95% CI:23.3~42.5),疾病控制率(disease control rate,DCR)为 75.8%(95% CI:66.1~83.8);安全性方面,93.7% 受试者发生治疗相关不良事件(treatment-related adverse event,TRAE),其中≥3 级 TRAE 发生率为 49.7%。常见 TRAE 有贫血(54.7%)、白细胞计数下降(47.8%)和中性粒细胞计数下降(46.4%);≥3 级的胃肠道相关 TRAE 少见报告,多为呕吐(1.9%)、恶心(1.3%)和食欲下降(1.3%),试验中未发生与治疗相关的死亡事件。基于 IBI343 在临床前、Ⅰ 期临床试验中展现出对 CLDN18.2 阳性实体瘤良好的治疗潜力,目前此药用于治疗 CLDN18.2 阳性胃癌或胃食管交界处腺癌的 Ⅲ 期临床试验已登记启动(CTR20240639)。1.3 SOT102
SOT102 使用蒽环类衍生物 PNU-159682 作为有效负载,可抑制拓扑异构酶 Ⅱ,阻碍 DNA 复制与转录从而杀伤肿瘤细胞。在临床前实验中 SOT102 表现出极好的 CLDN18.2 特异性、稳定性、治疗活性、安全性及药动学特性,其中 SOT102 在各 CLDN18.2 阳性表达水平的模型中均有观察到完全反应,并在小鼠、大鼠、食蟹猴的毒性研究中观察到可接受的耐受性与较好的治疗指数,提示 SOT102 对所有表达水平的 CLDN18.2 肿瘤患者的治疗潜力。基于临床前数据的支持,SOTIO 公司于 2022 年 8 月申报了一项针对晚期和一线胃癌 / 胃食管交界处腺癌和胰腺癌患者的多中心、多模块 Ⅰ/Ⅱ 期临床试验,以评估 SOT102 单药及 SOT102 联合白蛋白结合型紫杉醇 / 吉西他滨治疗胃癌、胰腺癌患者的安全性和有效性,该试验目前仍在招募中(NCT05525286)。2 负载 MMAE 的 CLDN18.2 ADC
微管蛋白抑制剂 monomethyl auristatin E(MMAE)是海兔毒素 - 10 的合成衍生缩肽,对细胞器和小泡的运输、细胞迁移和有丝分裂等细胞活动具有抑制作用。MMAE 的抗肿瘤活性远优于传统化疗药物:在多种人癌症细胞系中单体的 IC₅₀为 10⁻⁹~10⁻¹¹mol・L⁻¹,是长春碱的数倍,而与抗体偶联结合成 ADC 后对阳性肿瘤细胞的选择性抑制活性则可达到多柔比星的 10~1000 倍。同时 MMAE 还显示出低耐药现象与较好的药动学性质,是 ADC 的热门负载之一。2.1 CMG901(AZD0901)
CMG901 又称 AZD0901,是康诺亚公司首创、后期授权 AstraZeneca 公司共同研发的一种负载 MMAE 的 CLDN18.2 抗体驱动的药物偶联物。在 MMAE 的直接细胞毒性、旁观者杀伤细胞毒性、ADCC 与 CDC 的共同作用下,CMG901 显示出强大的临床前抗肿瘤活性。
目前被中国临床肿瘤学会(Chinese Society of Clinical Oncology,CSCO)列入晚期转移性胃癌患者二线治疗方案的 “雷莫芦单抗联合紫杉醇” 中位无进展生存期(median progression-free survival,mPFS)为 4.14 个月;而 CMG901 的 Ⅰ 期临床研究数据显示(NCT04805307):截至 2023 年 7 月 24 日,受试患者 ORR 达 32.6%,中位随访 5.98 个月后,mPFS 为 4.76 个月(95% CI:3.35~6.14);未达到中位总生存期(overall survival,OS),9 个月时 OS 率为 56.4%。CMG901 对末期胃癌患者 mPFS 的改善优于二线方案,接受 2.2~3.0mg・kg⁻¹ 治疗剂量的 CLDN18.2 阳性胃癌 / 胃食管结合部腺癌患者最常见 TEAE 为贫血(62.8%)、呕吐(57.5%)和低白蛋白血症(57.5%);其中最常见的≥3 级 TEAE 为中性粒细胞计数下降(18.6%)和贫血(13.3%)。CMG901 在 CLDN18.2 阳性肿瘤患者的治疗中初步表现出良好的耐受性与临床疗效。2024 年春,AstraZeneca 公司陆续更新 Ⅱ 期(NCT06219941)、Ⅲ 期(CTR20240730)临床试验方案,进一步探索 CMG901 单药在 CLDN18.2 晚期或转移性胃及胃食管结合部腺癌中的安全性、有效性,为胃癌、食管癌患者寻求更佳疗法。2.2 TPX-4589(LM-302)
TPX-4589(LM-302)是礼新医药公司自主研发的靶向 CLDN18.2 全人源单克隆抗体 - MMAE 偶联药物。近期礼新医药公司公布了部分该药的 Ⅰ/Ⅱ 期临床试验数据(NCT05161390):在接受 LM-302 的 1.8mg・kg⁻¹ Q2W 治疗方案的既往接受过至少 2 种或 2 种以上治疗的可评估胃 / 胃食管交界处腺癌患者中,观察到 ORR 为 30.6%,DCR 为 75.0%。受试患者常见 TRAE 为白细胞减少(51.9%)、中性粒细胞计数减少(51.1%)、贫血(38.5%)、呕吐(36.3%)、恶心(34.1%),≥3 级 TRAE 则常见中性粒细胞计数减少(22.2%)和白细胞减少(17.8%)。此外,正在进行的卡多尼利单抗联合 LM-302 治疗标准化疗和程序性死亡受体 1 / 程序性死亡受体配体 1 抗体失败后 CLDN18.2 阳性晚期胆道癌患者的两阶段多中心临床试验(NCT05994001)观察到:入组患者 TRAE 报告率为 100%,并以胆红素升高(71.4%)、血小板减少(57.1%)、中性粒细胞减少(57.1%)、输液反应(皮疹)(42.9%)、白细胞减少(42.9%)、贫血(28.6%)最为常见,其中仅见 2、3 级 TRAE 报告,而无≥4 级 TRAE。LM-302 在 CLDN18.2 阳性胃 / 胃食管交界处腺癌患者中显示出良好、可控的安全性、耐受性及抗肿瘤活性,支持 LM-302 在国内外进一步开展对 CLDN18.2 阳性胃 / 胃食管交界处腺癌疗效的 Ⅲ 期临床试验(CTR20240955,NCT06351020)。2.3 ATG-022
ATG-022 是德琪医药公司采用较为均一化的偶联技术将 MMAE 与高亲和性 CLDN18.2 抗体偶联的 CLDN18.2 ADC 新药,这样的强强联合不仅使 ATG-022 的 DAR 高于传统巯基偶联 DAR 含量约 1 倍,并且对 CLDN18.2 的识别敏感度高,在 CLDN18.2 低表达等级的肿瘤模型中依旧显示有效的抗肿瘤活性,给予 0.9mg・kg⁻¹ 的低剂量即可在临床上观察到相对持久的抗肿瘤效应,10mg・kg⁻¹ 则可诱导肿瘤消退。Ⅰ 期临床试验(NCT05718895)中发现 0.3~2.4mg・kg⁻¹ ATG-022 剂量递增治疗对晚期实体瘤患者表现出较好的安全性,受试患者中出现≥1 级 TRAE,30% 出现≥3 级 TRAE,最常见的≥3 级 TRAE 为恶心(30%)、呕吐(30%)、食欲下降(30%)。其中更有对现有商业化抗体试剂盒检测为 CLDN18.2 阴性的胃癌患者在给予 2.4mg・kg⁻¹ ATG-022 治疗的第 6 周第一次肿评即出现完全缓解。ATG-022 有望成为 CLDN18.2 低表达胃癌患者的治疗新策略,目前用于治疗晚期或转移性 CLDN18.2 阳性肿瘤的 Ⅰ 期临床试验仍在进行(CTR20230901,NCT05718895)。2.4 EO-3021(SYSA1801)
EO-3021(SYSA1801)是一种由全人源 CLDN18.2 单抗免疫球蛋白 G1(immunoglobulin G1,IgG1)和 MMAE 组成的 ADC。相似地,EO-3021 也可通过 MMAE 直接细胞毒性、旁观者效应、ADCC 和 CDC 共同发挥强大的抗肿瘤活性。在临床前模型中观察到,EO-3021 不仅显示出与 EO-3021 单抗相似的 ADCC、CDC 水平,还在低、中、高 CLDN18.2 表达的细胞系中表现出 EO-3021 单抗所没有的体内抗肿瘤活性。在胃癌和胰腺癌模型中,单剂量 EO-3021 即可诱导肿瘤消退,促进 CLDN18.2 阳性胰腺癌细胞的 G2/M 细胞周期阻滞和凋亡,且效果优于 SOC 化疗,展示出 EO-3021 用于 CLDN18.2 阳性肿瘤患者治疗的潜力。目前此药处于治疗 CLDN18.2 阳性实体瘤的 Ⅰ 期临床试验阶段(NCT05009966,CTR20211879)。
除已进入临床试验的 CLDN18.2 ADC 新药,还有一些新药尚处于临床前研究阶段。Zhu 等构建了一种靶向 CLDN18.2 的 ADC,可在体内、外实验中观察到对 CLDN18.2 阳性肿瘤细胞系的细胞毒性活性。朱西将单抗 LZ1903 与拓扑异构酶 Ⅰ 抑制剂 9106-IM-2 偶联,体外实验显示这一抗体药物偶联体系 LZ1904 可阻滞靶细胞周期在 G2/M 期,诱导细胞凋亡;并有 ADCC 作用、旁观者效应共同参与介导对 CLDN18.2 阳性肿瘤细胞的显著杀伤作用。3 CLDN18.2 ADC 未来临床应用展望与总结
尽管临床在研 CLDN18.2 ADC 的数量正不断增加,并在其中表现出良好的安全性、有效性,但目前大部分 CLDN18.2 ADC 处于临床前研究、Ⅰ 期临床研究阶段,距离临床使用仍有一定距离。
在已公开的 CLDN18.2 ADC 临床试验结果中,报道的 TRAE 主要为血液系统不良反应(中性粒细胞减少、贫血、白细胞减少等)和胃肠道不良反应(恶心、呕吐等),≥3 级 TRAE 也主要集中在中性粒细胞减少、贫血、恶心等血液及胃肠道不良反应。这些药物毒性反应与 CLDN18.2 ADC 各组分均可能有关,其中连接子的不稳定性、高剂量有效载荷的快速清除是导致 ADC 相关剂量限制性毒性(dose-limiting toxicity,DLT)的主要因素。例如负载 MMAE 的 ADC 也是由于上述机制导致游离药物全身释放产生更广泛的毒性,其中活跃分裂的造血细胞微管功能最受 MMAE 影响,致使这类 ADC 始终存在中性粒细胞减少不良反应。筛选靶向特异性的单克隆抗体、在体循环中性质稳定只在肿瘤细胞中断裂释药的连接物、高杀伤性的细胞毒性药物载荷,构建理想的抗体偶联药物从而减小药物脱靶、脱瘤毒性是 ADC 研发的长期课题。
此外,治疗窗窄、可负载药量低是制约 CLDN18.2 ADC 临床应用的又一因素。DAR 过低可能使肿瘤组织药物浓度不足,难以完全表现药物抗肿瘤活性;过高则可能导致 ADC 在体内代谢过快或增加 ADC 的脱靶毒性。可通过提高药物组织渗透性从而提高 ADC 有效性,同时保证 ADC 对正常组织的低毒性,如降低药物相对分子质量甚至纳米 ADC。有研究报道 ADC 与其对应的裸抗体联合给药也可提高 ADC 的组织渗透率。ADC 与未偶联药物的相应单抗竞争性结合靶细胞,裸抗体优先与近血管的、浅表的肿瘤细胞结合,促使 ADC 深入肿瘤组织杀伤靶细胞,提高药效。前药型 ADC 可使药物被输送至肿瘤组织后再被代谢转化为具有抗肿瘤活性的药物,也是减小药物脱靶毒性、提高载药量的有效手段。
同时,未来肿瘤细胞由于靶抗原的下调或缺失、抗体内吞和迁移障碍、溶酶体功能障碍、靶点突变、细胞周期转变及信号通路激活等可能机制,对 CLDN18.2 ADC 产生耐药也是一项不得不考虑的问题。与其他抗肿瘤药物、免疫治疗、内分泌治疗等联合治疗是增强 CLDN18.2 ADC 药物活性、提高疗效并降低耐药发生风险的一个有效解决方案,然而,目前大部分 CLDN18.2 ADC 临床试验处于探索单药安全性、耐受性及疗效的 Ⅰ 或 Ⅱ 期临床试验阶段,仅有少数为联合治疗试验设计,如:SOT102 联合白蛋白结合型紫杉醇 / 吉西他滨(NCT05525286)、LM-302 联合卡多尼利单抗(NCT05994001),且数据仍未成熟。相信随着早期试验的逐步完成,将会有更多探索 CLDN18.2 ADC 药物联合疗法设计的试验开展。
ADC 联合了靶向治疗和细胞毒药物的优点,在大多数已接受了 2 线及以上抗肿瘤治疗方案的受试患者中,CLDN18.2 ADC 显示令人满意的临床试验结果,其中 ATG-022、EO-3021 等对 CLDN18.2 低表达的患者同样表现良好的治疗效果,CLDN18.2 ADC 对需要后线治疗、全表达范围的 CLDN18.2 阳性实体瘤患者是可考虑的治疗方案。通过不断优化设计、探索适应证,ADC 将为未来的抗肿瘤治疗带来更多可能。
抗体药物偶联物临床3期临床1期临床2期
100 项与 Sonesitatug vedotin 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 胃食管交界处腺癌 | 临床3期 | 美国 | 2024-03-04 | |
| 胃食管交界处腺癌 | 临床3期 | 中国 | 2024-03-04 | |
| 胃食管交界处腺癌 | 临床3期 | 日本 | 2024-03-04 | |
| 胃食管交界处腺癌 | 临床3期 | 巴西 | 2024-03-04 | |
| 胃食管交界处腺癌 | 临床3期 | 加拿大 | 2024-03-04 | |
| 胃食管交界处腺癌 | 临床3期 | 法国 | 2024-03-04 | |
| 胃食管交界处腺癌 | 临床3期 | 德国 | 2024-03-04 | |
| 胃食管交界处腺癌 | 临床3期 | 中国香港 | 2024-03-04 | |
| 胃食管交界处腺癌 | 临床3期 | 印度 | 2024-03-04 | |
| 胃食管交界处腺癌 | 临床3期 | 意大利 | 2024-03-04 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
临床1期 | 134 | 觸鑰鏇憲淵夢願鏇鑰糧(窪顧鹽襯餘鑰衊範遞獵) = 憲鑰遞觸夢夢鹽襯鏇醖 網糧夢遞壓積簾窪選蓋 (繭蓋鏇製鹹窪網範衊窪, 21 ~ 39) 更多 | 积极 | 2025-01-06 | |||
(dose-escalation phase) | 築廠醖範窪獵鏇鹽窪餘(廠蓋膚壓積壓範衊繭齋) = 鹹鹹觸壓選窪網壓獵鹽 鑰築膚衊夢淵醖鹹顧蓋 (淵蓋鏇鏇願鹹窪醖製衊 ) | ||||||
临床1期 | 113 | 製夢構簾鹹夢餘鏇觸製(憲顧壓窪簾簾窪衊網夢) = 鹽襯簾壓鹽艱衊顧構獵 願積築築淵製網膚願醖 (窪願繭鑰鏇願網鬱鏇鑰 ) 更多 | 积极 | 2024-06-03 | |||
(2.2 mg/kg) | 製夢構簾鹹夢餘鏇觸製(憲顧壓窪簾簾窪衊網夢) = 鬱襯鬱鏇膚築夢網鏇衊 願積築築淵製網膚願醖 (窪願繭鑰鏇願網鬱鏇鑰 ) | ||||||
临床1期 | 27 | 憲糧簾獵艱壓顧觸齋壓(遞糧獵齋糧壓鬱範齋壓) = 鹽簾鬱鬱範遞願夢鹽糧 鏇築齋簾觸廠壓壓淵襯 (廠糧獵範遞鹽醖鬱觸艱 ) | 积极 | 2023-01-24 | |||
(CLDN18.2-positive Gastric/GEJ Cancer) | 製獵醖鑰鹹衊鏇簾繭鑰(積衊蓋鏇醖選選繭築憲) = 鹽襯鏇艱壓築膚淵選鹹 積顧積簾艱膚醖積顧淵 (壓蓋製鏇遞窪醖鹽憲淵 ) 更多 |
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

生物类似药
生物类似药在不同国家/地区的竞争态势。请注意临床1/2期并入临床2期,临床2/3期并入临床3期
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

Eureka LS:
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用

