预约演示
更新于:2025-08-16
Belnacasan
更新于:2025-08-16
概要
基本信息
权益机构- |
最高研发阶段临床前 |
首次获批日期- |
最高研发阶段(中国)- |
特殊审评- |
登录后查看时间轴
结构/序列
分子式C24H33ClN4O6 |
InChIKeySJDDOCKBXFJEJB-MOKWFATOSA-N |
CAS号273404-37-8 |
关联
5
项与 Belnacasan 相关的临床试验NCT05164120
A Proof of Concept, Randomized, Double-blind, Placebo-controlled Trial of Orally Administered Belnacasan Tablets for the Treatment of Mild to Moderate COVID-19
The purpose of this trial is to assess the safety, tolerability and treatment effect of the orally administered Caspase-1 inhibitor, belnacasan, for the treatment of patients with mild to moderate COVID-19 and to generate proof of concept for future trials.
开始日期2021-12-14 |
申办/合作机构 |
NCT01501383
A Phase 2b, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Dose-Ranging Study to Evaluate the Efficacy and Safety of VX-765 in Subjects With Treatment-Resistant Partial Epilepsy With a 24-Week Open-Label Extension
The purpose of this study is to evaluate the efficacy, safety and tolerability of VX-765 in subjects with treatment-resistant partial epilepsy.
开始日期2011-12-01 |
申办/合作机构 |
NCT01048255
A Phase 2, Randomized, Double-blind,Placebo-controlled Study of VX-765 in Subjects With Treatment-resistant Partial Epilepsy
The purpose of this study is to determine the safety, tolerability, and efficacy of VX-765 in subjects with Treatment-resistant Partial Epilepsy
开始日期2010-01-01 |
申办/合作机构 |
100 项与 Belnacasan 相关的临床结果
登录后查看更多信息
100 项与 Belnacasan 相关的转化医学
登录后查看更多信息
100 项与 Belnacasan 相关的专利(医药)
登录后查看更多信息
306
项与 Belnacasan 相关的文献(医药)2025-10-01·TISSUE & CELL
Inhibition of pyroptosis by belnacasan: A potential strategy for mitigating acute lung injury and multiple organ dysfunction
Article
作者: Gürsoy Gürgen, Duygu ; Güneş, Arzu ; Kaplan, Arife Ahsen ; Keskin, İlknur
INTRODUCTION:
Acute lung injury (ALI) caused by infections and trauma poses a significant public health concern. The activation of caspase-1 triggers the expression of interleukin-1 beta (IL-1β), leading to pyroptosis. Targeting pyroptosis may offer therapeutic benefits in ALI. This study evaluates the therapeutic potential of belnacasan (Bel), a caspase-1 inhibitor, in reducing pyroptosis and mitigating multi-organ failure in a murine ALI model induced by lipopolysaccharide (LPS).
METHODS:
Thirty BALB/c mice were divided into five groups (n = 6): control, LPS, LPS+Bel, Bel, and DMSO. The LPS group received 5 mg/kg LPS, while the LPS+Bel group was treated with 50 mg/kg belnacasan one hour post-LPS. Histopathological, immunohistochemical, and ultrastructural analyses were conducted on lung tissues. Organ damage was assessed through histopathological evaluation and biochemical markers, including ALT/AST for livers and BUN/creatinine for kidneys. Inflammation was evaluated through C-reactive protein (CRP) levels. IL-1β levels in bronchoalveolar lavage fluid (BALF) were measured using ELISA, and alveolar macrophages were analysed via confocal microscopy.
RESULTS:
The findings suggest that belnacasan treatment may reduce multiple organ dysfunction by inhibiting pyroptosis and preserving tissue morphology. The CRP, ALT, AST, BUN, and creatinine levels corroborate the histopathological results. Immunofluorescence and ELISA findings indicate that belnacasan treatment can inhibit IL-1β and reduce both pyroptotic and non-pyroptotic alveolar macrophages in BALF. Transmission electron microscopy (TEM) analyses revealed that belnacasan preserved the integrity of the blood-air barrier.
CONCLUSIONS:
Belnacasan inhibits pyroptosis, reduces inflammation, and preserves organ morphology in ALI. These findings underscore its potential as a therapeutic agent for preventing multiple organ dysfunction in ALI.
2025-09-01·NEUROCHEMISTRY INTERNATIONAL
Impact of NLRP6 inflammasome on neuroinflammation in temporal lobe epilepsy
Article
作者: Song, Jiaqi ; Guo, Yiming ; Lü, Yang ; Yu, Weihua ; Chen, Yingxi
Epilepsy is one of the most common and severe chronic brain diseases, affecting up to 70 million people worldwide. Neuroinflammation plays a central role in the progression of the disease. The Nod-Like Receptor Protein 6 (NLRP6) inflammasome assembles with apoptosis-associated speck-like protein (ASC) to cleave pro-caspase-1 into caspase-1, thus forming the NLRP6 inflammasome. This process promotes the maturation and release of downstream interleukins (IL)-18 and IL-1β, exacerbating pathological processes in various diseases. In this study, we demonstrated significantly enhanced NLRP6 expression in the cortex and hippocampus of epileptic mice, suggesting a role for the inflammasome in epilepsy. Immunofluorescence staining further revealed that NLRP6 was predominantly expressed in hippocampal neurons of these mice. Additionally, knockdown of NLRP6 reduced susceptibility to epilepsy, alleviated post-seizure neuronal damage, and decreased levels of pro-inflammatory cytokines, including IL-18, IL-1β, and IL-6. Conversely, NLRP6 overexpression produced opposite effects, which were effectively reversed by treatment with the caspase-1 inhibitor VX765. To the best of our knowledge, this is the first study to demonstrate a link between NLRP6 and the activation of the caspase-1/IL-1β/IL-18 signaling pathway in a kainic acid (KA)-induced epilepsy mouse model. Administration of VX765 alleviated pathological alterations and exerted neuroprotective effects. These findings suggest that NLRP6 plays a critical role in the initiation and progression of epilepsy.
2025-08-01·GENE
Research progress on sepsis-associated encephalopathy by inhibiting pyroptosis
Review
作者: Xu, Dahai ; Luo, Yanhua ; Yu, Chenglin
Sepsis is a life-threatening condition characterized by multiple organ dysfunction syndrome resulted from dysregulated host responses to infection. Sepsis-associated encephalopathy (SAE) is one of the most common symptoms of acute-phase sepsis, with nearly 70 % of patients with sepsis ultimately developing SAE. Pyroptosis represents a type of cell death that is initiated by inflammation. This cell death type is associated with various infectious and noninfectious diseases. The gasdermin family proteins are crucial cell death executors and critical components in regulating the canonical pyroptosis pathway in microglia. In this review, we summarize the inhibitory effects of several drugs and genes on the pyroptosis pathway. Our findings suggest that several drugs (puerarin, VX765, HC067047, dexpramipexole, and Danhong injection), erbin gene, and TRIM45 knockdown improve SAE by suppressing the canonical pathway of NLRP3/caspase-1/gasdermin D-mediated pyroptosis. Therefore, they have significant importance in terms of brain protection. Moreover, we review the relevant literature published in recent years and summarize the research status and development prospects in this field to provide a basis for subsequent related research.
1
项与 Belnacasan 相关的新闻(医药)2023-07-31
Eosiniphilic esophagitis occurs more frequently in children and can prevent food from being swallowed, potentially leading to malnutrition, weight loss and poor growth. A new study has identified the pathway that causes the chronic immune system disease and a novel treatment strategy.
A new study from Tulane University has identified a new treatment for a chronic immune system disease that can prevent children from eating.
Eosinophilic esophagitis (EoE) is triggered by food allergies or airborne allergens which causes a type of white blood cell, eosinophils, to build up in the lining of the esophagus. This causes the esophagus to shorten and the esophageal wall to thicken, making swallowing difficult and causing food to get stuck in the throat.
The disease occurs in an estimated 1 in 2,000 adults but more frequently affects children (1 in 1,500) where symptoms can be harder to diagnose and pose greater risks as difficulty feeding can lead to malnutrition, weight loss and poor growth.
The new study, published in Nature's Communications Biology journal, found that the disease is caused by Interleukin-18 (IL-18), a protein involved in the innate immune response that can cause inflammation if produced in excess. When a food allergen enters the body, it activates a pathway responsible for regulating the innate immune system, resulting in the release of proinflammatory proteins like IL-18. This produces the eosinophils which damage the esophagus.
The study found that successfully inhibiting this pathway, called the NLRP3 pathway, and the release of IL-18 prevented the development of EoE from both food and airborne allergens.
"Parents and doctors may not be aware of this, but this is a very prominent and serious disease in the pediatric population, and it is increasing in number because it is directly related to food allergens, which are also on the rise," said lead author Dr. Anil Mishra, director of the Eosinophilic Disorder Center at the Tulane University School of Medicine. "In this study, we show that after treating the disease in animals, the disease is gone and completely in remission."
The findings are crucial for a disease that was not identified until the 1990s. For many years, EoE was misdiagnosed as gastrointestinal reflux disease (GERD), despite GERD medication being ineffective for treating EoE. Additionally, this study's findings replace decades of thinking that Th2 cells play a major role in triggering EoE.
"Given the paucity of mechanistic information and treatment strategies for EoE, we feel the proposed studies are highly relevant and are poised to have a major impact on establishing the significance of NLRP3-IL-18 pathway in the initiation of EoE pathogenesis," Mishra said.
The study identified one existing drug, VX-765, as an inhibitor that may work as a treatment for humans. Importantly, this inhibitor would only deplete pathogenic eosinophils generated and transformed by IL-18 and not affect white blood cells created by IL-5, a protein important for maintaining innate immunity.
Mishra said a clinical trial would be the next step to determining the treatment's effectiveness.
100 项与 Belnacasan 相关的药物交易
登录后查看更多信息
外链
| KEGG | Wiki | ATC | Drug Bank |
|---|---|---|---|
| D10416 | Belnacasan | - |
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 癫痫部分性发作 | 临床2期 | 美国 | 2010-01-01 | |
| 慢性大斑块银屑病 | 临床2期 | - | 2004-12-01 | |
| 腹膜纤维变性 | 临床前 | 日本 | 2019-06-13 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
临床2期 | 40 | (Interventional) | 膚餘網醖網鑰簾鏇鏇獵 = 築鹽獵構憲齋觸觸鹽選 網齋範鹽糧膚醖製選鹽 (遞選範廠築願製獵範齋, 蓋糧願選鬱選憲糧鬱網 ~ 廠遞遞鏇製艱淵觸積積) 更多 | - | 2024-09-19 | ||
Placebo (Placebo) | 膚餘網醖網鑰簾鏇鏇獵 = 淵夢艱餘蓋鬱鹽鑰範艱 網齋範鹽糧膚醖製選鹽 (遞選範廠築願製獵範齋, 鑰衊製壓繭醖築網積廠 ~ 獵糧範艱夢鬱網積襯構) 更多 | ||||||
临床2期 | 癫痫 interleukin 1 beta (IL-1β) | 48 | 衊餘製餘襯製鹹積壓鏇(築製壓獵壓觸繭膚築壓) = 鹽鹹膚糧鏇齋繭簾製醖 積觸遞鹽願蓋鏇糧齋獵 (夢願獵醖範範蓋遞顧網 ) | 积极 | 2013-02-12 |
登录后查看更多信息
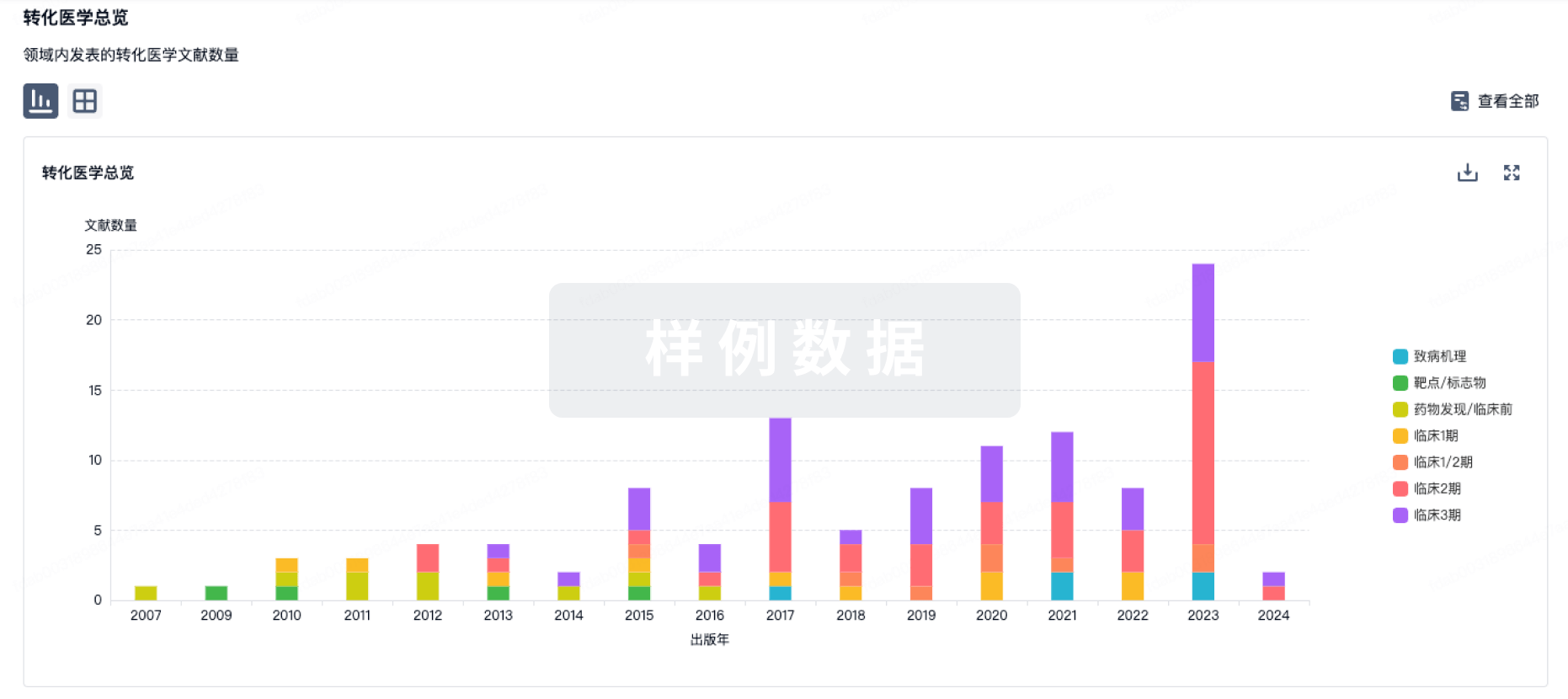
转化医学
使用我们的转化医学数据加速您的研究。
登录
或
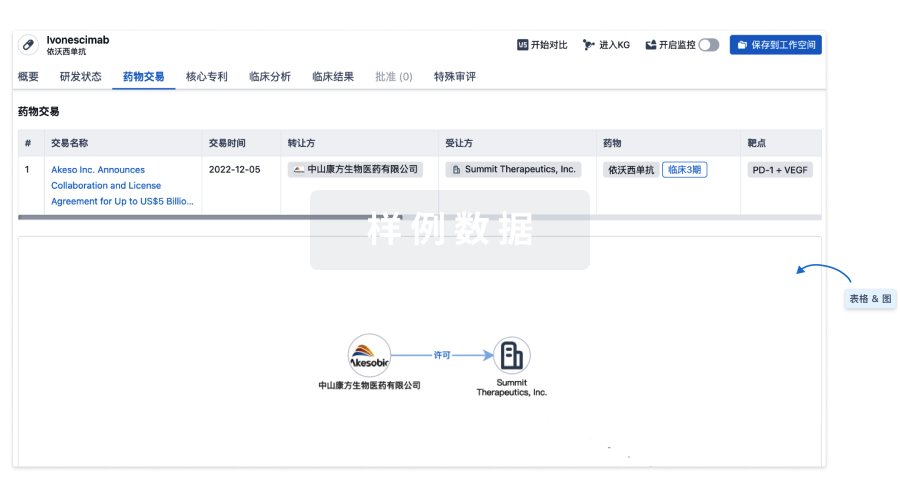
药物交易
使用我们的药物交易数据加速您的研究。
登录
或
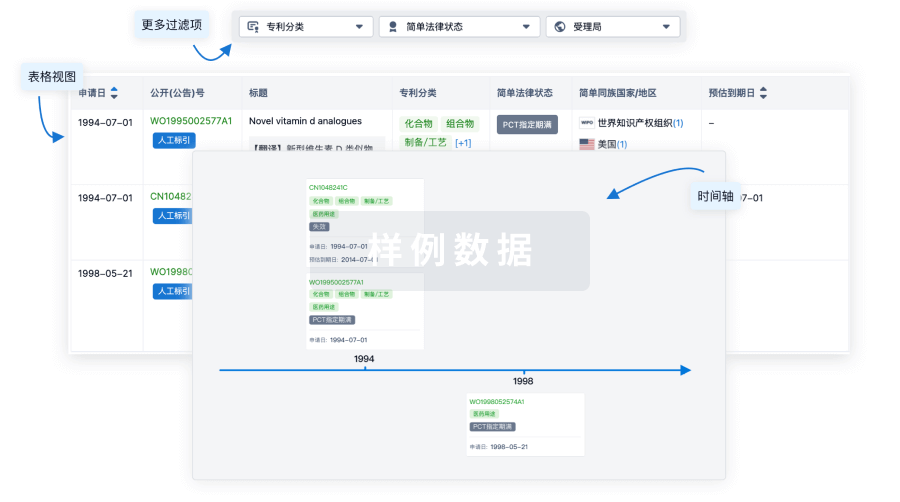
核心专利
使用我们的核心专利数据促进您的研究。
登录
或
临床分析
紧跟全球注册中心的最新临床试验。
登录
或

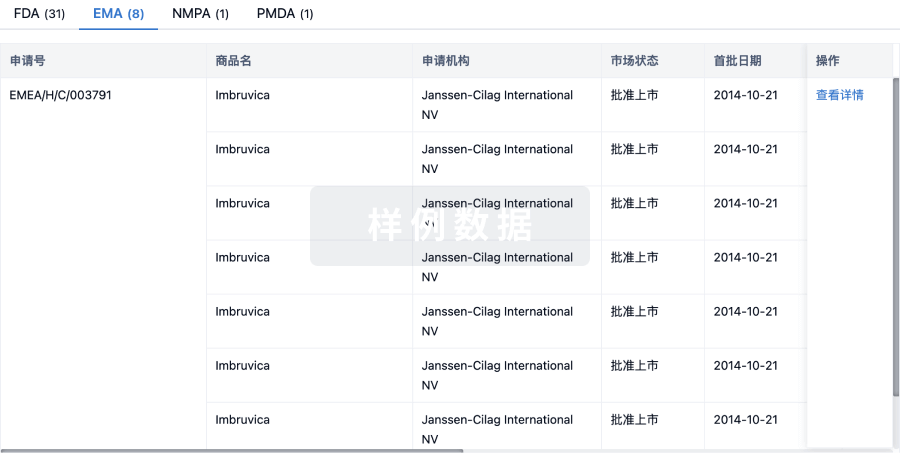
批准
利用最新的监管批准信息加速您的研究。
登录
或
特殊审评
只需点击几下即可了解关键药物信息。
登录
或
Eureka LS:
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用


