预约演示
更新于:2025-09-24
ALE-P02
更新于:2025-09-24
概要
基本信息
非在研机构- |
权益机构- |
最高研发阶段临床1/2期 |
首次获批日期- |
最高研发阶段(中国)- |
特殊审评快速通道 (美国) |
登录后查看时间轴
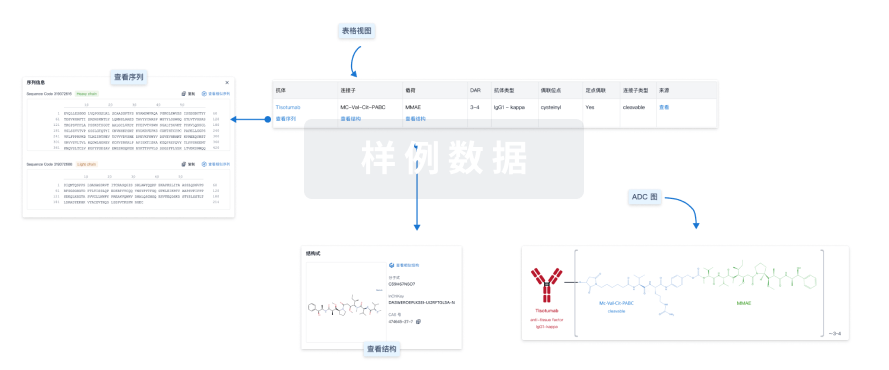
结构/序列
使用我们的ADC技术数据为新药研发加速。
登录
或
关联
1
项与 ALE-P02 相关的临床试验NCT06747585
A Phase I/II, Open-Label, Multicenter Study of ALE.P02 (Claudin-1 Targeted Antibody-Drug Conjugate) as a Monotherapy in Adult Patients With Selected Advanced or Metastatic CLDN1+Squamous Solid Tumors
The purpose of this study is to evaluate the safety, tolerability, pharmacokinetic, pharmacodynamic, preliminary anti-tumor activity, and to determine the recommended Phase II dose (RP2D) of the ALE.P02 monotherapy in adult patients with selected squamous solid tumors.
开始日期2024-12-16 |
申办/合作机构 |
100 项与 ALE-P02 相关的临床结果
登录后查看更多信息
100 项与 ALE-P02 相关的转化医学
登录后查看更多信息
100 项与 ALE-P02 相关的专利(医药)
登录后查看更多信息
10
项与 ALE-P02 相关的新闻(医药)2025-09-23
·药明康德
日前,业界知名的年度生物技术猛公司(Fierce Biotech’s 2025 Fierce 15)榜单火热出炉。这一榜单旨在展现业界具有创新精神的生物技术公司。上榜的公司极具多元性,所开发的治疗模式包含细胞疗法、小分子药物、蛋白降解疗法等,亦包含癌症、肥胖、神经退行性疾病等多种适应症。下面让我们来了解一下今年上榜的生物医药新锐。
新锐公司:Alentis Therapeutics
成立年份:2019
临床专攻:Claudin-1(CLDN1)阳性实体瘤和器官纤维化
Alentis自2019年创立以来,便持续获得稳定融资支持:首轮募资1250万美元,2023年再获1.05亿美元;最新D轮达到1.81亿美元,由Novo Holdings、OrbiMed与Jeito Capital等知名生命科学投资方领投。在研发管线方面,Alentis聚焦新颖靶点Claudin-1,同步推进肿瘤与纤维化两条主线:一是以抗体偶联药物(ADC)瞄准鳞状细胞癌等实体瘤;二是面向器官瘢痕化(包括肝纤维化)开展治疗探索。核心项目lixudebart(原ALE.F02)旨在逆转肾、肝、肺等多器官纤维化,适应症涵盖ANCA相关性血管炎、进展期肝纤维化和特发性肺纤维化,当前处于1/2期临床阶段。肿瘤方面,Alentis正推进面向Claudin-1阳性实体瘤的潜在“first-in-class”ADC候选疗法ALE.P02与ALE.P03,其中ALE.P02已获FDA快速通道资格(适用于不限癌种的Claudin-1阳性肿瘤),两款候选药物均处于1/2期临床试验。
新锐公司:Alterome Therapeutics
成立年份:2021
临床专攻:乳腺癌、子宫内膜癌、结直肠癌、胰腺导管腺癌、非小细胞肺癌、实体瘤
Alterome Therapeutics以精准医学为核心,聚焦胰腺癌、结直肠癌、肺癌等具有高度未满足医疗需求的领域。公司依托对致癌通路的深入理解,结合基于化学结构与物理学的快速设计方法(Kraken计算化学平台)与高效执行,在约三年半内完成从概念到临床的转化。公司目前管线中两款候选药物均处于1期临床:选择性KRAS抑制剂ALTA3263力求在保持高选择性的同时,对几乎所有KRAS突变实现持久抑制,已在结直肠癌、胰腺癌与非小细胞肺癌(NSCLC)人群中开展研究;共价AKT1 E17K突变选择性抑制剂ALTA2618旨在仅靶向致病突变、避免影响野生型蛋白,正于激素受体阳性乳腺癌、子宫内膜癌与卵巢癌等人群中接受评估。公司计划先获取两项单药数据,随后加速推进联合治疗并探索潜在注册路径。Alterome于2024年完成由Goldman Sachs Alternatives领投、总额1.32亿美元的B轮融资,为后续临床推进提供资金支持。
新锐公司:Character Biosciences
成立年份:2019
临床专攻:眼科疾病
Character Biosciences以数据与遗传学驱动的平台为基础,聚焦干性年龄相关性黄斑变性(AMD)等眼科疾病,通过五年自然史研究构建高质量患者数据并据此确定靶点与分层人群,形成“更早干预、防止进展”的治疗范式。其两项核心管线分别为进入1期的潜在“first-in-class”脂质调节剂CTX203,以及拟在一年内进入临床、旨在显著减缓视网膜细胞死亡与视力丧失的补体抑制剂CTX114;两者均由平台验证、针对明确的遗传致病通路。公司与博士伦(Bausch + Lomb)合作开展AMD新药发现,并在2024年完成1.10亿美元B轮融资(参与方包括Sanofi Ventures),为推进两款候选药物至概念验证奠定资金基础。
新锐公司:Congruence Therapeutics
成立年份:2021
临床专攻:罕见疾病
Congruence Therapeutics是一家位于蒙特利尔的计算驱动型生物技术公司,由Clarissa Desjardins博士与Rocky Goldsmith博士共同创建,该公司构建以生物物理模型为核心的药物发现平台。这一平台可系统描绘蛋白折叠的多构象状态,从中发现潜在结合位点,并通过虚拟筛选评估小分子化合物是否可以通过与这些位点结合纠正蛋白构象,恢复功能。该公司同时与药企合作,将能力延展至非错误折叠靶点。管线方面,公司计划最早于2025年底向加拿大监管机构提交首个针对遗传性肥胖的小分子候选药物的临床试验申请,并在次年提名用于治疗帕金森病亚型和α1-抗胰蛋白酶缺乏症的两项新候选药物。该公司在2023年3月完成6500万美元A轮融资,为临床推进提供保障。
新锐公司:Formation Bio
成立年份:2016
临床专攻:溃疡性结肠炎、膝关节骨性关节炎、慢性手部湿疹
Formation Bio并非传统意义上的新药发现型公司,而是以临床试验这一研发中最长、最昂贵的环节作为切入点,致力于以更高效率推动项目跨越概念验证(PoC)节点,打造由人工智能(AI)赋能的新一代大型医药公司。公司由Benjamine Liu博士与Linhao Zhang博士共同创立,前身为专注以技术手段降低试验成本与周期的TrialSpark;在此基础上,企业逐步转型为“自研与引进并行”的管线运营模式。Formation Bio此前完成3.72亿美元C轮融资,用于获取处于准备进入临床试验但尚未获得PoC数据的候选药物,并通过快速推进取得关键数据,然后再依据结果将药物授权给合作伙伴进一步开发。今年6月与赛诺菲(Sanofi)达成的5.45亿欧元授权合作即为一例。依托包括Muse在内的AI工具,公司可大幅压缩以往需数月完成的患者招募与机构审查委员会(IRB)合规材料准备过程。该公司同时并行推进10–15个资产的组合式布局,以形成可复制的效率优势并分散单项风险。
新锐公司:Georgiamune
成立年份:2019
临床专攻:癌症和自身免疫性疾病的免疫治疗
Georgiamune围绕免疫功能失衡这一根源性问题展开布局,力求通过精准调控免疫系统,一方面扩大肿瘤免疫治疗的受益人群,另一方面为自身免疫性疾病提供更有针对性的干预手段。公司已将三项潜在“first-in-class”候选药物推进至临床:GIM-407用于治疗自身免疫性疾病,采用淋巴吸收途径选择性激活调节性T细胞(Treg),以避免药物血液暴露,从而降低全身性免疫抑制与感染风险;GIM-531针对实体瘤,通过抑制Treg活性增强机体抗肿瘤免疫,现正开展单药及联合抗PD-1抗体的临床研究;GIM-122为靶向T细胞表位的全新抗体,可在抑制免疫检查点蛋白功能同时激活T细胞,旨在解决因缺乏活化T细胞而难以从免疫检查点抑制剂治疗中获益的患者的需求。
新锐公司:Incyclix Bio
成立年份:2020
临床专攻:卵巢癌和乳腺癌
Incyclix Bio由在周期蛋白依赖性激酶(CDK)领域合作17年的三位科学家——Patrick Roberts博士、Jay Strum博士与John Bisi先生——共同创立。他们在G1 Therapeutics公司的工作推动了CDK4/6抑制剂Cosela(trilaciclib)的获批。Incyclix Bio专注以更高效、更具选择性的CDK2抑制剂应对肿瘤对CDK4/6抑制剂治疗的耐药性。公司核心候选药物INX-315在早期临床前研究中显示出抑制CDK2–cyclin E的强力活性。在适应症布局上,Incyclix Bio同时在卵巢癌(含CCNE1扩增人群)与乳腺癌领域开展研究:INX-315在卵巢癌扩展剂量队列中已观察到42%的客观缓解率;在乳腺癌中则正推进与氟维司群及Verzenio等方案的联合治疗。
新锐公司:Nuvig Therapeutics
成立年份:2022
临床专攻:慢性自身免疫性和炎症性疾病
Nuvig Therapeutics针对慢性自身免疫病领域中,以静脉注射免疫球蛋白(IVIG)为标准治疗时,所面临的高剂量输注、时间成本与供应不确定等痛点,推出以重组Fc片段为核心的候选药物NVG-2089。该疗法通过选择性结合II型Fc受体,激活机体内源性抗炎通路,实现免疫调节而非免疫抑制,并以约1/10至1/20的剂量重现IVIG的关键免疫调节效应,从而在安全性与便利性上具备潜在优势。公司已在炎性脱髓鞘性多发性神经病(CIDP)患者中进行NVG-2089的全球性2期INVGOR研究,并正在评估将该疗法拓展至免疫性血小板减少症及自身免疫性大疱性皮肤病。Nuvig已完成由Novo Holdings与Platanus领投的4700万美元A轮融资,并在随后完成1.61亿美元B轮融资。
新锐公司:Ouro Medicines
成立年份:2024
临床专攻:免疫介导疾病的T细胞衔接器
Ouro Medicines的目标是用工程化T细胞衔接器(T-cell engager)替代长期免疫抑制方案,以提升疗效与药物可及性。该公司由Monograph Capital与GSK共同孵化,在今年初走出隐匿模式,并获1.2亿美元资金支持。首席执行官Jaideep Dudani博士曾主导Hi-Bio项目布局,现有多位Hi-Bio骨干加入这一约20人的团队。其主打药物OM336是一款BCMA/CD3靶向双特异性抗体,通过调节抗体对CD3受体的亲和力来拓宽治疗窗。在1/2期研究的分析中,相较已批准骨髓瘤疗法,该药物的重度细胞因子释放综合征发生率更低。目前该公司已启动OM336在两类自身免疫性细胞减少症中的1b期篮子研究(美国与澳大利亚入组),并获FDA孤儿药资格用以治疗自身免疫性溶血性贫血(AIHA)。
新锐公司:Seaport Therapeutics
成立年份:2024
临床专攻:神经精神疾病
Seaport Therapeutics由PureTech Health创始人Daphne Zohar女士所创立,她及其团队在公司创立半年内便完成两轮合计3.25亿美元融资。该公司旗下Glyph平台通过经淋巴系统递送提升口服生物利用度、减少肝脏代谢与所致毒性,从而具潜力将已具人体有效性证据但因安全性受限而未能上市的“旧分子”转化为可用新药。公司聚焦神经精神疾病管线,主打管线GlyphAllo(SPT-300)已在针对抑郁症患者的2b期试验当中完成首位患者给药,并在广泛性焦虑障碍患者中开展1期试验。该公司同时进行临床前研究以推进其另一款在研小分子SPT-348用于难治性抑郁与头痛。
新锐公司:Trace Neuroscience
成立年份:2024
临床专攻:神经退行性疾病,尤其是肌萎缩侧索硬化(ALS)
Trace Neuroscience由Eric Green博士与Aaron Gitler博士等学者共同创立,他们于2022年在《自然》发表研究,指出TDP-43功能缺失会导致ALS相关基因UNC13A错配剪接,进而产生非功能性蛋白。基于这一机制,公司主打项目TRCN-1023为一反义寡核苷酸(ASO)疗法,旨在修复因TDP-43缺陷引发的UNC13A错配剪接、恢复关键突触蛋白表达,旨在覆盖不携带SOD1变异,从而难以从现有获批疗法中获益的广泛ALS人群。2024年11月,公司完成1.01亿美元A轮融资,计划于明年启动临床。
新锐公司:TrimTech Therapeutics
成立年份:2023
临床专攻:帕金森病、亨廷顿病和阿尔茨海默病
TrimTech Therapeutics以TRIMTAC这一新型蛋白降解策略切入神经退行性疾病等高难度领域。该公司主要基于剑桥大学MRC分子生物学实验室与英国痴呆研究院的研究,聚焦于E3连接酶TRIM21,旨在通过双功能性小分子降解剂与分子胶两种策略靶向并清除积累的蛋白聚集体,可潜在应用于治疗亨廷顿病、阿尔茨海默病与帕金森病。TrimTech于2024年完成3100万美元种子轮融资,公司计划利用该笔资金推进体内概念验证与早期去风险分析,并以A轮融资与主打项目的临床数据获取为目标,实现从机制验证到临床转化的顺畅衔接。
新锐公司:Triveni Bio
成立年份:2023
临床专攻:湿疹、哮喘和潜在其他炎症疾病
Triveni Bio由Amagma Therapeutics与Modify Therapeutics于2023年合并成立,名称寓意“三河汇流”,对应其三大原则:以新生物学解锁疗效、人类遗传学指导的靶点开发、以抗体为核心的技术平台。在免疫与炎症(I&I)领域中,公司以差异化机制切入,强调在既有治疗基础上提升疗效上限。管线方面,主打项目TRIV-509为抑制皮肤中丝氨酸蛋白酶KLK5/7的单克隆抗体,意在通过“屏障修复+直接抗炎/止痒”的双路径用于特应性皮炎治疗,已启动首次临床试验。同时,TRIV-573作为下一代双特异性抗体,通过抑制KLK5/7与IL-13信号通路,力求在维持皮肤屏障的同时进一步降低Th2炎症。公司已先后完成A轮9200万美元与B轮1.15亿美元融资,为后续临床推进与管线拓展提供支持。
新锐公司:Umoja Biopharma
成立年份:2019
临床专攻:血液肿瘤、自身免疫性疾病和实体瘤
Umoja Biopharma专注于开发体内CAR-T疗法。该公司由创始人兼首席执行官Andy Scharenberg博士领导,目标是加速从研发走向临床验证。其技术平台采用体内慢病毒递送,实现对T细胞的持久改造;在达到CAR-T早期扩增峰值的同时,通过激活细胞因子受体,力求获得更深、更持久的临床应答。这一思路在递送方式与持久性上形成差异化,为提升疗效与应用可及性提供了明确方向。在管线推进与合作方面,公司与艾伯维(AbbVie)合作的CD19项目UB-VV111已在美国与澳大利亚进入早期临床开发。此外,Umoja与驯鹿生物合作布局CD22项目,当前重点聚焦肿瘤领域,并同步探索自身免疫适应症。公司在对外合作上保持弹性,但核心目标明确——推动体内CAR-T走向自主商业化。
新锐公司:Verdiva Bio
成立年份:2025
临床专攻:肥胖和心脏代谢疾病
Verdiva凭借在肥胖代谢领域的差异化口服治疗方案受到关注:今年1月该公司以4.1亿美元A轮融资亮相,并自Sciwind Biosciences引进3款减重药物。其中进展最快的为每周一次给药的口服GLP-1受体激动剂,计划于年内启动2期研究并在2026年获得初步数据。与此同时,每周一次口服胰淀素受体激动剂预计明年启动临床试验。基于其口服候选药物,Verdiva可在固定剂量复方药物中灵活调整两者比例,在耐受性与疗效之间寻求更好的平衡。该公司预计在2026年前将第四个未披露项目推进至临床阶段,进一步丰富开发管线。
参考资料:
[1] Introducing Fierce Biotech's 2025 Fierce 15. Retrieved September 22, 2025 from https://www.fiercebiotech.com/special-reports/introducing-fierce-biotechs-2025-fierce-15
免责声明:本文仅作信息交流之目的,文中观点不代表药明康德立场,亦不代表药明康德支持或反对文中观点。本文也不是治疗方案推荐。如需获得治疗方案指导,请前往正规医院就诊。
版权说明:欢迎个人转发至朋友圈,谢绝媒体或机构未经授权以任何形式转载至其他平台。转载授权请在「药明康德」微信公众号回复“转载”,获取转载须知。
分享,点赞,在看,聚焦全球生物医药健康创新
抗体药物偶联物临床1期快速通道
2025-03-13
自2011年以来,瑞士连续13年全球创新指数排名第一,是全球重要的创新策源地,也是中国首个创新战略伙伴关系国,在生命健康、先进制造、低碳科技等领域与中国具有极佳互补性。
BioBAY与Insight Tech共建的S²CUBE(中瑞产业协同创新中心),旨在以生命科学为核心领域,推动瑞士创新技术、创新企业、创新人才及创新生态圈与BioBAY及园区企业的长期交流与合作。
S²CUBE将定期发布介绍瑞士生命健康领域的前沿创新项目,促进BioBAY及园区企业与瑞士创新的双边技术交流、双向产业对接和创新合作引进。本期介绍的Alentis Therapeutics是2023年《瑞士创新100强》上榜企业,其致力于开发Claudin-1抗体及管线。
图源Businesswire
瑞士生物科技公司Alentis Therapeutics(以下简称Alentis)成立于2019年,该公司首创专门针对Claudin-1蛋白靶点的单克隆抗体。该抗体能阻断细胞纤维化信号,打开僵硬的细胞外基质,引导免疫系统和免疫疗法攻击肿瘤,并在癌症中选择性杀死肿瘤细胞,提供了治疗器官纤维化和CLDN1阳性肿瘤的开创性疗法。
Alentis以斯特拉斯堡大学Thomas Baumert教授实验室、法国国立卫生研究院(INSERM)病毒和肝病研究所、HepSYS卓越实验室、斯特拉斯堡大学医院研究所以及其他合作者的创新研究为基础,由Thomas Baumert教授成立。
图源Baselarea
实体瘤约占目前成人癌症的90%,尽管早期检测和治疗技术有所进步,但肿瘤患者的整体生存率提升并不明显。这是因为肿瘤患者癌症细胞内的Claudin-1过度表达并暴露在紧密连接之外,暴露的Claudin-1会驱动细胞外基质重塑形成物理屏障,使肿瘤阻挡免疫系统的攻击,并对免疫疗法药物产生抵抗。
图源Alentis Therapeutics官网
Alentis首次将在器官纤维化与癌症中起着关键作用的Claudin-1蛋白作为靶点,开发了专门针对Claudin-1的单克隆抗体。该抗体能打开僵硬的细胞外基质,引导免疫疗法和免疫系统攻击肿瘤。
Claudin-1是构成细胞之间紧密连接的蛋白质的组成部分,其重要作用是使上皮细胞连接在一起。在健康细胞中,Claudin-1隐藏在紧密连接内。但在纤维化组织和许多癌症中,Claudin-1会过度表达并暴露在紧密连接之外,驱动细胞外基质成分过度积累,形成物理屏障来阻挡免疫系统攻击肿瘤,并导致纤维化器官衰竭。
Alentis专门针对暴露在紧密连接外的Claudin-1,首创开发出仅与其结合的单克隆抗体,以抑制暴露的Claudin-1发挥作用,从而破坏肿瘤生长并逆转纤维化进程。目前,Alentis已开发出系列Claudin-1抗体管线,包括用于治疗CLDN1阳性实体瘤的ALE.C04和用于治疗肝、肾和肺纤维化的Lixudebart。
ALE.C04会选择性地与暴露了Claudin-1的肿瘤细胞相结合,通过其效应功能 (ADCC) 杀伤肿瘤细胞,同时破坏肿瘤周围的胶原屏障,恢复免疫细胞运输,使免疫系统可以有效攻击和杀死肿瘤细胞。目前,ALE.C04已被FDA列入快速通道计划,用于治疗Claudin-1阳性头颈部鳞状细胞癌(HNSCC),正在进行1/2期临床试验。HNSCC是全球第六大常见癌症,最常发生在口腔、咽喉和喉头,其病例数和死亡率始终居高不下,暴露的Claudin-1通常出现在此类肿瘤上。
Lixudebart主要针对肾脏、肝脏和肺纤维化,特别是针对ANCA相关血管炎(AAV)、晚期肝纤维化和特发性肺纤维化。伴有肾脏损害的AAV是一种罕见、严重且可能致命的疾病。在高达85%的AAV病例中,肾脏在发病后2年内会逐渐出现纤维化,以致严重或完全丧失肾功能。特发性肺纤维化是一种进展性肺病,患者确诊后平均可存活2.5-5年。Lixudebart会选择性地结合暴露的Claudin-1,来阻断细胞内的纤维化信号,并破坏暴露的Claudin-1和胶原结合受体之间的物理相互作用,以打开细胞外基质形成的屏障,保持或恢复器官功能。该抗体目前正在进行二期临床试验。
除了常规疗法,Alentis正在推进的临床前项目还包括抗体-药物偶联物(ADC)、双特异性抗体和T细胞接合剂等下一代抗CLDN1疗法。其中,ALE.P02和ALE.P03是针对CLDN1阳性肿瘤的ADC管线,可以将治疗癌症的有效负载与抗体准确偶联,不致影响到其他健康组织。目前,ALE.P02正在接受GLP毒理学研究,并计划开展首次人体临床试验;ALE.P03正计划进行GLP毒理学研究。
2023年春,Alentis完成了1.05亿美元的C轮融资,该笔资金将帮助Alentis获取更多的临床数据并进一步推进其单抗管线的开发。作为针对Claudin-1开发癌症和纤维化潜在治疗方法的全球领先公司,Alentis致力于释放Claudin-1作为治疗靶点的广阔潜力,为患者带来创新的治疗方法。
▌文章来源:招商部
责编:何文正
审核:任旭
推荐阅读
S²CUBE丨瑞士生物科技公司ArcoScreen开发GPCR原代细胞筛选平台,基于微流控技术识别G蛋白偶联受体
S²CUBE丨瑞士生物科技公司Recolony开发结直肠癌细菌疗法,通过补充患者缺少的特定微生物菌株激活免疫细胞再生
S²CUBE丨瑞士医疗科技公司Artiria Medical研发可实时变形转向的导丝探针,改善神经介入手术疗效
免疫疗法
2025-01-09
Robust Dose-Dependent Target Engagement Demonstrated by Lixudebart in Both Studies
Preliminary Evidence of Improvement in Organ Function: eGFR and Proteinuria in ANCA-RPGN, and ALT/AST in Liver Fibrosis
Lixudebart Exhibited a Favorable Safety Profile and was Well-Tolerated
BASEL, Switzerland I January 09, 2025 I
Alentis Therapeutics (“Alentis”), a clinical-stage biotechnology company developing treatments for Claudin-1 positive (CLDN1+) tumors and organ fibrosis, announced positive topline results from two clinical trials of lixudebart (ALE.F02), a monoclonal antibody targeting Claudin-1 (CLDN1), developed to reverse organ fibrosis.
Lixudebart was investigated in two double-blind, placebo-controlled, randomized studies. RENAL-F02 is an ongoing Phase 2 study in Anti-Neutrophil Cytoplasmic Antibody (ANCA) Associated Vasculitis (AAV) with Rapidly Progressive Glomerulonephritis (RPGN) (
NCT06047171
), where 26 patients have been dosed for up to 24 weeks. FEGATO-01 is a Phase 1b clinical trial where 41 patients with mainly advanced F3/F4 liver fibrosis and/or mild cirrhosis were dosed for up to 4 weeks (
NCT05939947
).
“
The results from the two studies are very encouraging, particularly given the dose-dependent target engagement and favorable safety profile,
”
said Luigi Manenti, Chief Medical Officer.
“
We also observed an initial decrease in ALT/AST in liver fibrosis patients treated with lixudebart.”
“Interim results from the RENAL-F02 study in ANCA-RPGN indicate beneficial effects of lixudebart on Glomerular Filtration Rate (GFR) recovery and proteinuria reduction,”
said David Jayne, Professor of Clinical Autoimmunity at Cambridge University
.
“
These observations were further supported by reductions of CD163, a validated urinary biomarker, also indicating an impact on immune cell homing and trafficking to the kidney.”
In both clinical trials, lixudebart demonstrated a favorable safety profile, both as a monotherapy and in combination with standard of care (n=51 active patients across the two trials). These findings are consistent with the safety results from the Phase 1 clinical trial of lixudebart in healthy volunteers (n=48 active patients).
About Lixudebart (ALE.F02)
Lixudebart is a first-in-class monoclonal antibody developed for liver, lung and kidney fibrosis. The investigational antibody is designed to reverse organ fibrosis by specifically targeting a unique CLDN1 epitope exposed in fibrotic tissue. In Phase 1 single- and multiple-ascending dose studies in healthy volunteers, lixudebart was well tolerated, with no serious safety concerns. Lixudebart has been granted Orphan Drug designation by the FDA for the treatment of Idiopathic Pulmonary Fibrosis (IPF).
About Alentis Therapeutics
Alentis Therapeutics is a clinical-stage biotechnology company pioneering first-in-class antibody-drug conjugates (ADCs) and antibodies targeting Claudin-1 (CLDN1) for oncology and multi-organ fibrosis. CLDN1 is a previously unexploited target that plays a key role in the pathology of cancer and fibrotic disease. Alentis is the leading company pioneering CLDN1 ADCs and antibodies to modify and reverse the course of select diseases. The FDA granted Fast Track designation to the lead ADC program, ALE.P02, for the treatment of advanced or metastatic CLDN1+ squamous cancers, irrespective of the organ of origin.
Alentis was founded based on ground-breaking research in the laboratory of Prof. Thomas Baumert, MD at the University of Strasbourg and the French National Institute of Health and Medical Research (Inserm). Alentis is headquartered at the pharma-biotech hub in Basel, Switzerland, with an R&D subsidiary in Strasbourg, France, and clinical operations in the US. Visit
www.alentis.ch
SOURCE:
Alentis Therapeutics
临床结果孤儿药快速通道临床1期临床2期
100 项与 ALE-P02 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 宫颈鳞状细胞癌 | 临床2期 | 美国 | 2024-12-16 | |
| 宫颈鳞状细胞癌 | 临床2期 | 法国 | 2024-12-16 | |
| 宫颈鳞状细胞癌 | 临床2期 | 中国香港 | 2024-12-16 | |
| 宫颈鳞状细胞癌 | 临床2期 | 意大利 | 2024-12-16 | |
| 宫颈鳞状细胞癌 | 临床2期 | 新加坡 | 2024-12-16 | |
| 宫颈鳞状细胞癌 | 临床2期 | 韩国 | 2024-12-16 | |
| 宫颈鳞状细胞癌 | 临床2期 | 西班牙 | 2024-12-16 | |
| 宫颈鳞状细胞癌 | 临床2期 | 中国台湾 | 2024-12-16 | |
| 食管鳞状细胞癌 | 临床2期 | 美国 | 2024-12-16 | |
| 食管鳞状细胞癌 | 临床2期 | 法国 | 2024-12-16 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
No Data | |||||||
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

生物类似药
生物类似药在不同国家/地区的竞争态势。请注意临床1/2期并入临床2期,临床2/3期并入临床3期
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用
