预约演示
更新于:2025-09-01
Apazunersen
更新于:2025-09-01
概要
基本信息
非在研机构- |
最高研发阶段临床3期 |
首次获批日期- |
最高研发阶段(中国)- |
特殊审评突破性疗法 (美国)、快速通道 (美国)、孤儿药 (美国)、罕见儿科疾病 (美国)、孤儿药 (欧盟)、优先药物(PRIME) (欧盟)、孤儿药 (日本) |
登录后查看时间轴
结构/序列
使用我们的RNA技术数据为新药研发加速。
登录
或
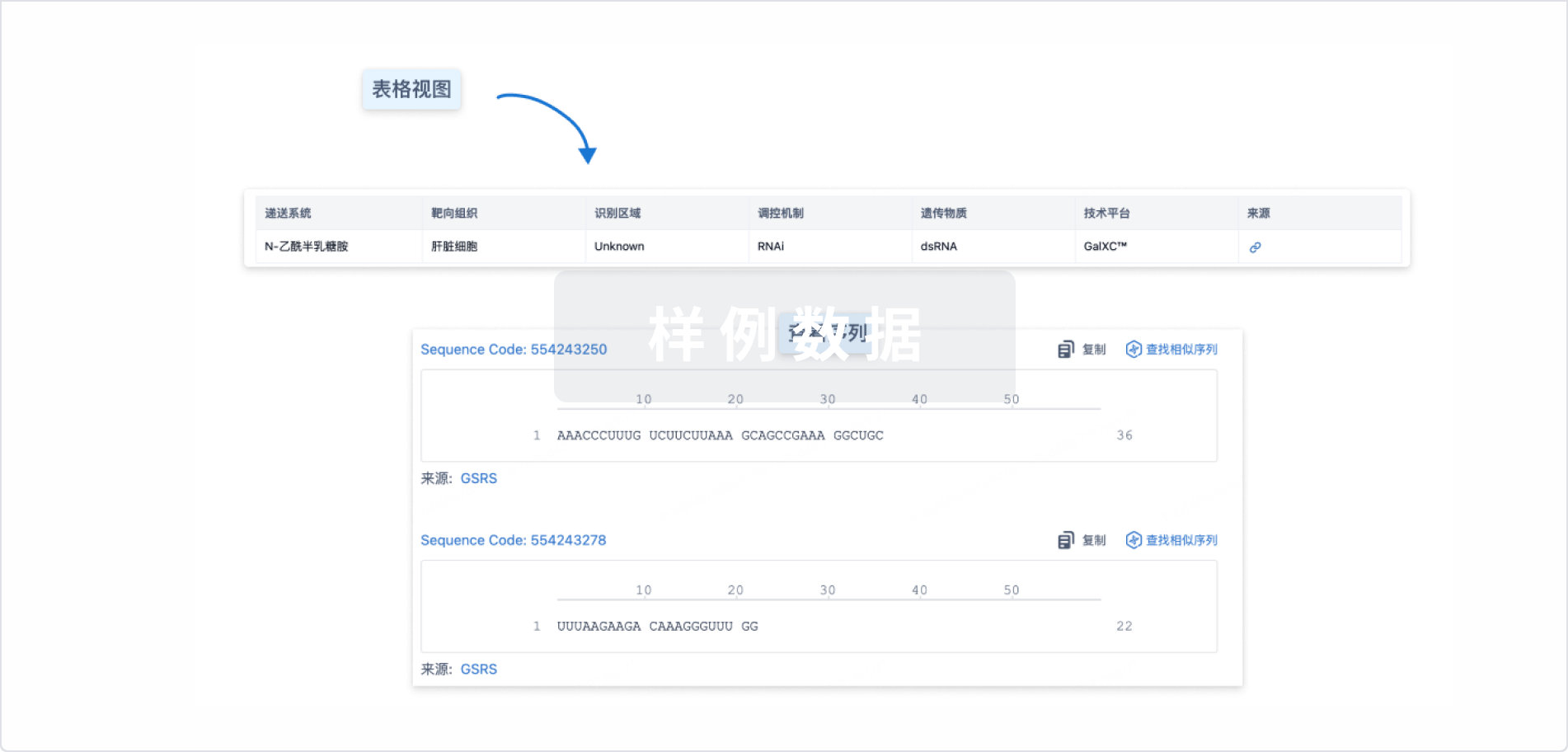
Sequence Code 1142932845

来源: *****
关联
3
项与 Apazunersen 相关的临床试验NCT06617429
A Phase 3, Randomized, Double-blind, Sham-controlled Study Investigating the Efficacy and Safety of GTX-102 in Pediatric Subjects With Angelman Syndrome
The primary objective of this study is to evaluate the effect of GTX-102 in cognitive function in participants with deletion-type Angelman Syndrome (AS).
开始日期2024-12-03 |
NCT06415344
A Long-term Extension Trial Investigating the Safety and Efficacy of GTX-102 in Patients With Angelman Syndrome
The primary objective of the study is to evaluate the long-term safety profile of GTX-102 in participants with Angelman Syndrome (AS)
开始日期2024-07-31 |
NCT04259281
A Phase 1/2 Open-label, Multiple-dose, Dose-escalating Clinical Trial of the Safety and Tolerability of GTX-102 in Pediatric Patients with Angelman Syndrome (AS)
The primary objective of the study is to evaluate the safety and tolerability of multiple-ascending doses of GTX-102 administered by intrathecal (IT) injection to participants with Angelman Syndrome (AS).
开始日期2020-02-24 |
100 项与 Apazunersen 相关的临床结果
登录后查看更多信息
100 项与 Apazunersen 相关的转化医学
登录后查看更多信息
100 项与 Apazunersen 相关的专利(医药)
登录后查看更多信息
1
项与 Apazunersen 相关的文献(医药)2021-05-01·NATURE BIOTECHNOLOGY
Slivers of the spectrum
作者: Branca, Malorye
Large-scale genomic studies are reinvigorating interest in a small group of molecularly defined autism-associated disorders and spurring renewed interest in genetic therapies.
57
项与 Apazunersen 相关的新闻(医药)2025-08-12
Submission of New Drug Application (NDA) to U.S. Food and Drug Administration (FDA) for GTx-104 Seeking Approval of GTx-104 in the Treatment of Patients with aneurysmal Subarachnoid Hemorrhage (aSAH)
NDA Supported by Data from Phase 3 STRIVE-ON Safety Trial, which Met Primary Endpoint and Provided Evidence of Clinical Benefit Compared to Orally Administered Nimodipine
PRINCETON, N.J., Aug. 12, 2025 (GLOBE NEWSWIRE) -- Grace Therapeutics, Inc. (Nasdaq: GRCE) (Grace Therapeutics or the Company), a late-stage, biopharma company advancing GTx-104, a clinical-stage, novel, injectable formulation of nimodipine being developed for IV infusion to address significant unmet medical needs in aSAH patients, today announced the financial results and business highlights for the quarter ended June 30, 2025.
“During our first quarter of 2026 we continued to execute on our clinical and corporate goals, led by the submission of our NDA to the FDA for GTx-104 for the treatment of aSAH,” said Prashant Kohli, CEO of Grace Therapeutics. “Our NDA for GTx-104 was a significant milestone for the Company and built on more than a decade of painstaking research and innovation. Our NDA is supported by a robust data package including positive results from our STRIVE-ON trial, which provided evidence of improved clinical outcomes in aSAH patients treated with GTx-104 as well as potential medical and pharmacoeconomic benefits of GTx-104 in the treatment of aSAH. The standard of care for aSAH has not seen meaningful innovation in nearly 40 years, and we believe the STRIVE-ON trial results point to a very promising role for GTx-104 as a potential breakthrough for the care of aSAH patients. We are hopeful the FDA will accept our NDA for formal review, and look forward to continued engagement with the FDA during the review process.”
First Quarter 2026 Corporate Highlights
Submission to the FDA of the Company’s NDA for GTx-104. The application included a comprehensive data package, including positive data obtained from the Company’s Phase 3 STRIVE-ON safety trial of GTx-104, whereby it met its primary endpoint and provided evidence of clinical benefit when compared to orally administered nimodipine.
Submission of the NDA has the potential to trigger the exercise of up to $7.6 million in warrants issued as part of a private placement the Company completed in September 2023. Under the terms of the September 2023 private placement, each warrant is exercisable for one share of common stock at an exercise price of $3.003 per share. These warrants are immediately exercisable and will expire on the earlier of (i) the 60th day after the date of the acceptance by the FDA of an NDA for the Company's product candidate GTx-104 or (ii) September 25, 2028.
The FDA typically has a 60-day period to determine if the Company’s NDA is complete and acceptable for filing. Grace has obtained Orphan Drug Designation from the FDA for GTx-104, which generally provides seven years of marketing exclusivity in United States upon FDA approval of the NDA. Additionally, the Company believes that its U.S. and international patent estate will provide additional marketing exclusivity for GTx-104.
First Quarter 2026 Financial Results
The Company reported a net loss of $3.4 million, or $0.21 per share, for the three months ended June 30, 2025, an increase of $0.8 million from the net loss of $2.6 million, or $0.24 per share, for the three months ended June 30, 2024. The increase in net loss was primarily due to a $1.9 million difference in the change in fair value of derivative warrant liabilities and a $0.7 million decrease in income tax benefit, partially offset by a $1.8 million decrease in research and development expenses and a $0.1 million decrease in general and administrative expenses.
Total research and development expenses were approximately $0.9 million for the three months ended June 30, 2025, compared to $2.7 million for the three months ended June 30, 2024. The decrease of approximately $1.8 million was primarily due to a $1.9 million decrease in research activities mainly due to completion of our GTx-104 pivotal Phase 3 STRIVE-ON safety clinical trial, offset in part by a $0.1 million increase in professional fees in connection with the preparation and submission of our NDA to the FDA.
General and administrative expenses were approximately $2.1 million for the three months ended June 30, 2025, a decrease of $0.1 million from $2.2 million for the three months ended June 30, 2024. The decrease was primarily a result of decreased legal, accounting, tax, audit and other professional fees related to the continuance and domestication completed in October 2024, offset in part by an increase in salaries and benefits due to merit increases, increases in other general and administrative expenses primarily due to costs for GTx-104 commercial assessment, and an increase in stock-based compensation due to the issuance of new stock option awards.
Cash Runway
As of June 30, 2025, cash and cash equivalents were $20.0 million, a net decrease of approximately $2.1 million compared to cash and cash equivalents of $22.1 million at March 31, 2025.
The private placement the Company completed in February 2025 included common warrants exercisable for shares of common stock (or pre-funded warrants in lieu thereof) at an exercise price of $3.395 per share. Each common warrant is immediately exercisable and will expire on the earlier of (i) the 60th day after the date the FDA approves the NDA for GTx-104 and (ii) September 25, 2028. Potential gross proceeds from the exercise of the February 2025 common warrants are $15.0 million.
The private placement the Company completed in September 2023 included common warrants exercisable for shares of common stock at an exercise price of $3.003 per share. Each common warrant is immediately exercisable and will expire on the earlier of (i) the 60th day after the date of the acceptance by the FDA of the NDA for GTX-104 or (ii) five years from the date of issuance. Potential gross proceeds from the exercise of the September 2023 common warrants are $7.6 million.
While the Company believes that current cash and cash equivalents provide cash runway through at least the next twelve months, the runway could extend into the second quarter of calendar 2027 if all of the common warrants issued in connection with the Company’s February 2025 and September 2023 private placements are exercised at the election of the investors.
About the STRIVE-ON Trial
The STRIVE-ON trial (NCT05995405) was a prospective, randomized open-label trial of GTx-104 compared with oral nimodipine in patients hospitalized with aSAH. 50 patients were administered GTx-104 and 52 patients received oral nimodipine. The primary endpoint was the number of patients with at least one episode of clinically significant hypotension reasonably considered to be caused by the drug, and additional secondary endpoints included safety, clinical, and pharmacoeconomic outcomes. The trial met its primary endpoint, with patients receiving GTx-104 observed to have a 19% reduction in at least one incidence of clinically significant hypotension compared to oral nimodipine (28% versus 35%). Other measures also favored or were comparable to GTx-104, including: 54% patients had relative dose intensity (RDI) of 95% or higher compared to only 8% on oral nimodipine, and 29% more patients had favorable functional outcomes at 90 days. In addition, there were fewer intensive care unit (ICU) readmissions, ICU days, and ventilator days for patients receiving GTx-104 versus oral nimodipine. Adverse events were comparable between the two arms and no new safety issues were identified with patients receiving GTx-104. All deaths in both arms of the trial were due to severity of the patient’s underlying disease. There were eight deaths on the GTx-104 arm compared to four deaths on the oral nimodipine arm. The survival status of one patient on the oral nimodipine arm was unknown. No deaths were determined to be related to GTx-104 or oral nimodipine.
About aneurysmal Subarachnoid Hemorrhage (aSAH)
aSAH is bleeding over the surface of the brain in the subarachnoid space between the brain and the skull, which contains blood vessels that supply the brain. A primary cause of such bleeding is the rupture of an aneurysm in the brain. The result is aSAH, a relatively uncommon type of stroke that accounts for about 5% of all strokes and an estimated 42,500 U.S. hospital treated patients.
About the Grace Therapeutics Asset Portfolio
GTx-104 is a clinical stage, novel, injectable formulation of nimodipine being developed for IV infusion in aSAH patients to address significant unmet medical needs. The unique nanoparticle technology of GTx-104 facilitates aqueous formulation of insoluble nimodipine for a standard peripheral IV infusion.
GTx-104 provides a convenient IV delivery of nimodipine in the Intensive Care Unit potentially eliminating the need for nasogastric tube administration in unconscious or dysphagic patients. Intravenous delivery of GTx-104 also has the potential to lower food effects, drug-to-drug interactions, and eliminate potential dosing errors. Further, GTx-104 has the potential to better manage hypotension in aSAH patients. GTx-104 has been administered in over 200 patients and healthy volunteers and was well tolerated with significantly lower inter- and intra-subject pharmacokinetic variability compared to oral nimodipine.
GTx-102 is a novel, concentrated oral-mucosal spray of betamethasone intended to improve neurological symptoms of Ataxia-Telangiectasia (A-T), for which there are currently no FDA-approved therapies. GTx-102 is a stable, concentrated oral spray formulation comprised of the gluco-corticosteroid betamethasone that, together with other excipients can be sprayed conveniently over the tongue of the A-T patient and is rapidly absorbed. The Company received written responses to its End of Phase 1 meeting in GTx-102 where the FDA made recommendations on the path toward an NDA. The FDA provided guidance on the design of a single pivotal efficacy and safety trial, including the neurological assessment scale for the primary endpoint, that could, with appropriate confirmatory evidence, support an NDA. The further development of GTx-102 has been deprioritized in favor of focusing on development of GTx-104. It is also possible that the Company may license or sell GTx-102.
GTx-101 is a non-narcotic, topical bio-adhesive film-forming bupivacaine spray designed to ease the symptoms of patients suffering with postherpetic neuralgia (PHN). GTx-101 is administered via a metered-dose of bupivacaine spray and forms a thin bio-adhesive topical film on the surface of the patient’s skin, which enables a touch-free, non-greasy application. It also comes in convenient, portable 30 ml plastic bottles. Unlike oral gabapentin and lidocaine patches, which are used for the treatment of PHN, the Company believes that the biphasic delivery mechanism of GTx-101 has the potential for rapid onset of action and continuous pain relief for up to eight hours. No skin sensitivity was reported in a Phase 1 trial. The further development of GTx-101 has been deprioritized in favor of focusing on development of GTx-104. It is also possible that the Company may license or sell GTx-101.
About Grace Therapeutics
Grace Therapeutics, Inc. (Grace Therapeutics or the Company) is a late-stage biopharma company with drug candidates addressing rare and orphan diseases. Grace Therapeutics’ novel drug delivery technologies have the potential to improve the performance of currently marketed drugs by achieving faster onset of action, enhanced efficacy, reduced side effects, and more convenient drug delivery. Grace Therapeutic’s lead clinical assets have each been granted Orphan Drug Designation by the FDA, which provides seven years of marketing exclusivity post-launch in the United States, and additional intellectual property protection with over 40 granted and pending patents. Grace Therapeutics’ lead clinical asset, GTx-104, is an IV infusion targeting aneurysmal Subarachnoid Hemorrhage (aSAH), a rare and life-threatening medical emergency in which bleeding occurs over the surface of the brain in the subarachnoid space between the brain and skull.
For more information, please visit: www.gracetx.com.
Forward-Looking Statements
Statements in this press release that are not statements of historical or current fact constitute "forward-looking statements" within the meaning of the U.S. Private Securities Litigation Reform Act of 1995, as amended, Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, and "forward-looking information" within the meaning of Canadian securities laws (collectively, "forward-looking statements"). Such forward-looking statements involve known and unknown risks, uncertainties, and other factors that could cause the actual results of Grace Therapeutics to be materially different from historical results or from any future results expressed or implied by such forward-looking statements. In addition to statements which explicitly describe such risks and uncertainties, readers are urged to consider statements containing the terms "believes," "belief," "expects," "intends," "anticipates," "estimates," "potential," "should," "may," "will," "plans," "continue," "targeted" or other similar expressions to be uncertain and forward-looking. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this press release. The forward-looking statements in this press release, including statements regarding, the Company’s cash runway, the future prospects of the Company’s GTx-104 drug candidate, the timing and outcome of the Company’s NDA submission for GTx-104, the Company’s belief that the data and regulatory packages as currently structured will be sufficient for submission of such NDA, GTx-104’s potential to bring enhanced treatment options to patients suffering from aSAH, GTx-104’s potential to be administered to improve the management of hypotension in patients with aSAH, the ability of GTx-104 to achieve a pharmacokinetic and safety profile similar to the oral form of nimodipine, GTx-104’s potential to achieve medical and pharmacoeconomic benefit, GTx-104’s commercial prospects, the future prospects of the Company’s GTx-102 drug candidate, GTx-102’s potential to provide clinical benefits to decrease symptoms associated with A-T, the timing and outcomes of a Phase 3 efficacy and safety trial for GTx-102, the timing of an NDA filing for GTx-102, the future prospects of the Company’s GTX-101 drug candidate, GTX-101’s potential to be administered to PHN patients to treat the severe nerve pain associated with the disease and any future patent and other intellectual property filings made by the Company for new developments are based upon Grace Therapeutics’ current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, including, without limitation: (i) the success and timing of regulatory submissions of the Phase 3 STRIVE-ON safety trial for GTx-104; (ii) regulatory requirements or developments and the outcome of the Company’s NDA application for GTx-104; (iii) changes to regulatory pathways; and (iv) legislative, regulatory, political and economic developments. The foregoing list of important factors that could cause actual events to differ from expectations should not be construed as exhaustive and should be read in conjunction with statements that are included herein and elsewhere, including the risk factors detailed in the "Special Note Regarding Forward-Looking Statements," "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" sections of the Company's Annual Report on Form 10-K for the fiscal year ended March 31, 2025, the Company’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2025 to be filed with the Securities and Exchange Commission (“SEC”) and other documents that have been and will be filed by Grace Therapeutics from time to time with the SEC and Canadian securities regulators. All forward-looking statements contained in this press release speak only as of the date on which they were made. Grace Therapeutics undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by applicable securities laws.
For more information, please contact:
Grace Therapeutics Contact:
Prashant KohliChief Executive OfficerTel: 609-322-1602Email: info@gracetx.comwww.gracetx.com
Investor Relations:
LifeSci AdvisorsMike MoyerManaging DirectorPhone: 617-308-4306Email: mmoyer@lifesciadvisors.com
---tables to follow---
GRACE THERAPEUTICS, INC.Condensed Consolidated Balance Sheets(Unaudited)
June 30,2025 March 31, 2025 (Expressed in thousands except share data) $ $ Assets
Current assets:
Cash and cash equivalents 20,005 22,133 Receivables 20 126 Prepaid expenses 500 453 Total current assets 20,525 22,712 Equipment, net 14 15 Intangible assets 41,128 41,128 Goodwill 8,138 8,138 Total assets 69,805 71,993
Liabilities and Stockholders’ equity
Current liabilities:
Trade and other payables 2,315 1,930 Total current liabilities 2,315 1,930
Derivative warrant liabilities 1,628 1,141 Deferred tax liability 2,312 2,312 Total liabilities 6,255 5,383
Commitments and contingencies
Stockholders’ equity:
Preferred stock, $0.0001 par value per share; 10,000,000 authorized; none issued and outstanding as of June 30, 2025 and March 31, 2025 — — Common stock, $0.0001 par value per share; 100,000,000 authorized; 13,828,562 and 13,718,106 shares issued and outstanding as of June 30, 2025 and March 31, 2025, respectively 1 1 Additional paid-in capital 293,636 293,334 Accumulated other comprehensive loss (6,038) (6,038)Accumulated deficit (224,049) (220,687)Total stockholders' equity 63,550 66,610 Total liabilities and stockholders’ equity 69,805 71,993
GRACE THERAPEUTICS, INC.Condensed Consolidated Statements of Loss and Comprehensive Loss(Unaudited)
Three months ended
June 30, 2025 June 30, 2024
(Expressed in thousands, except share and per share data) $ $
Operating expenses
Research and development expenses (955) (2,708)General and administrative expenses (2,135) (2,255)Loss from operating activities (3,090) (4,963)
Foreign exchange gain (loss) 10 (8)Change in fair value of derivative warrant liabilities (487) 1,395 Interest and other income, net 205 235 Other (expense) income, net (272) 1,622 Loss before income tax benefit (3,362) (3,341)
Income tax benefit — 724
Net loss and total comprehensive loss (3,362) (2,617)
Basic and diluted loss per share (0.21) (0.24)
Weighted-average number of shares outstanding 15,924,522 10,928,543
临床结果申请上市财报孤儿药临床1期
2025-07-05
·药明康德
▎药明康德近期,全球多肽和寡核苷酸(TIDES)领域迎来系列进展。礼来的穆峰达(替尔泊肽注射液)在中国获批新适应症,用于治疗成人肥胖患者的中度至重度阻塞性睡眠呼吸暂停(OSA)。信达生物的玛仕度肽注射液在中国获批上市,用于成人肥胖或超重患者的长期体重控制。Unnatural Products(UNP)公司宣布与免疫学公司argenx达成一项超15亿美元的合作,旨在针对“不可成药”靶点开发口服大环肽疗法。本文将节选其中部分重要进展做简单介绍,仅供读者参阅。替尔泊肽:在中国获批新适应症中国国家药监局(NMPA)官网公示显示,礼来(Eli Lilly and Company)的替尔泊肽注射液在中国获批新适应症,用于治疗成人肥胖患者的中度至重度阻塞性睡眠呼吸暂停。替尔泊肽可改善中度至重度阻塞性睡眠呼吸暂停肥胖成人患者的睡眠障碍。该适应症的使用需在控制饮食和增加运动基础上进行。礼来新闻稿表示,接受替尔泊肽治疗的成人患者平均减重20%,睡眠中每小时呼吸暂停低通气次数至少减少27次;在接受替尔泊肽治疗一年后,半数以上患者已不再有OSA相关症状。替尔泊肽是一款葡萄糖依赖性促胰岛素多肽(GIP)和胰高糖素样肽-1(GLP-1)双受体激动剂药物。替尔泊肽针对肥胖根源发挥作用,可以减少食欲和饮食摄入。此前,替尔泊肽已获NMPA批准适用于:在饮食控制和运动基础上,接受二甲双胍和/或磺脲类药物治疗血糖仍控制不佳的成人2型糖尿病患者;在控制饮食和增加运动基础上,初始身体质量指数(BMI)符合以下要求的成人的长期体重管理:≥28 kg/㎡(肥胖),或≥24 kg/㎡(超重)并伴有至少一种体重相关合并症(例如高血压、血脂异常、高血糖、阻塞性睡眠呼吸暂停、心血管疾病等)。玛仕度肽:在中国获批上市NMPA宣布批准信达生物申报的胰高血糖素(GCG)/GLP-1双受体激动剂玛仕度肽注射液上市,用于成人肥胖或超重患者的长期体重控制。该药品适用于在控制饮食和增加体力活动基础上对成人患者的长期体重控制,初始BMI为:BMI≥28 kg/㎡(肥胖);或BMI≥24 kg/㎡(超重),并伴有至少一种体重相关的合并症(例如高血糖、高血压、血脂异常、脂肪肝、阻塞性睡眠呼吸暂停综合征等)。除了本次获批的适应症,玛仕度肽的第二项新药申请(NDA)正在NMPA受理审评中,用于成人2型糖尿病患者的血糖控制。玛仕度肽是是信达生物与礼来共同推进的一款GCG/GLP-1双受体激动减重药物,其具有独特的双激动作用机制,在激动GLP-1受体抑制食欲的基础上,同时激活GCG受体,促进脂肪燃烧,降低内脏脂肪含量,进一步增强玛仕度肽减重效果。临床研究表明,使用玛仕度肽体重降幅达21%,降低肝脏脂肪含量超80%,腰围减少达11 cm,颈围减少达3 cm,兼顾改善血糖、血压、血脂、血尿酸和转氨酶水平等心血管及代谢指标,带来全面获益。针对“不可成药”靶点开发口服大环肽疗法,新锐达成超15亿美元合作Unnatural Products公司宣布与免疫学公司argenx建立一项多靶点战略合作研究项目,旨在发现和开发针对“不可成药”疾病靶点的口服大环肽药物。该合作将利用UNP公司的专有药物发现平台,生成针对argenx公司所选定的多个靶点的高效、高选择性且可口服的大环肽分子。UNP将负责直到支持IND申请的临床前研究阶段,argenx将拥有针对多个适应症的这些靶点相关产品的开发和商业化的独家选择权。根据协议,UNP将获得数千万美元的预付款、近期里程碑付款以及研发资金支持。此外,UNP还有资格获得高达约15亿美元的潜在里程碑付款等款项。大环肽类药物兼具生物制品的精准性和小分子药物可口服给药的优势,同时能够作用于此前难以触及的细胞内靶点。UNP构建了一个可扩展的平台,使用人工智能(AI)引导设计、平行合成和直接生物学筛选来设计合成大环。GTX-102(apazunersen):获得美国FDA授予的突破性疗法认定Ultragenyx Pharmaceutical宣布,其在研反义寡核苷酸(ASO)疗法GTX-102(apazunersen)获得美国FDA授予的突破性疗法认定,用于治疗天使综合征(AS)。该认定主要基于一项1/2期临床试验的积极结果。该研究共纳入74名年龄在4至17岁之间、携带母源UBE3A基因完全缺失的天使综合征患者,结果显示,患者在接受治疗最长达3年后,在多个症状领域持续表现出快速且稳定的发育改善。GTX-102通过鞘内给药方式递送,该疗法可以促进神经元细胞中父系UBE3A的等位基因表达,产生患者体内所缺失的关键蛋白产物。该疗法的相关全球3期试验正在进行中。AX-0810:向EMA提交临床试验申请ProQR Therapeutics公司近日宣布已向欧洲药品管理局(EMA)提交临床试验申请(CTA),计划启动其候选疗法AX-0810的1期临床试验。AX-0810是一种在研ADAR介导的RNA编辑寡核苷酸(EON),通过GalNac递送系统靶向编码NTCP的mRNA。NTCP是一种将胆汁酸运输到细胞中的肝细胞蛋白。在胆汁淤积性疾病中,由于胆汁流动受阻,胆汁酸积累产生的毒性会引发进行性肝损伤。AX-0810通过选择性调节NTCP功能,减少胆汁酸再摄取到肝脏中,有望减轻炎症、纤维化并延缓肝衰竭进程。已有遗传学数据显示,天然存在的NTCP变异可以安全地降低胆汁酸再摄取,进一步支持该机制在改善肝脏健康和改变胆汁淤积性疾病病程方面的潜力。参考资料(可上下滑动查看)[1] 国家药监局批准玛仕度肽注射液上市. Retrieved July 4, 2025, from https://www.nmpa.gov.cn/zhuanti/cxylqx/cxypxx/20250627145044169.html[2] 穆峰达第三项适应症中国获批,填补睡眠呼吸暂停药物治疗领域空白. Retrieved July 4, 2025, from https://mp.weixin.qq.com/s/gP6nUnnNsoWRTVT78apUgA[3]中国国家药监局药品审评中心(CDE)官网. From https://www.cde.org.cn/main/xxgk/listpage/4b5255eb0a84820cef4ca3e8b6bbe20c[4] 舶望制药治疗慢性乙型肝炎创新药BW-20507与聚乙二醇干扰素 α 联合疗法获国家药监局II期临床试验许可. Retrieved July 4, 2025, from https://mp.weixin.qq.com/s/p55LVzcGVCINL_JM304opg[5] [1]圣因生物靶向CFB用于补体相关疾病的siRNA药物完成首例受试者给药. Retrieved July 4, 2025, from https://mp.weixin.qq.com/s/D_eVtaEYcIY89Vy7oALTNQ[6] Ultragenyx Receives Breakthrough Therapy Designation for GTX-102 in Angelman Syndrome. Retrieved June 27, 2025 from https://www.globenewswire.com/news-release/2025/06/27/3106516/20739/en/Ultragenyx-Receives-Breakthrough-Therapy-Designation-for-GTX-102-in-Angelman-Syndrome.html[7] ProQR Announces CTA Submission for Phase 1 Study of AX-0810 Targeting NTCP. Retrieved July 4, 2025, from https://www.globenewswire.com/news-release/2025/06/26/3106239/33039/en/ProQR-Announces-CTA-Submission-for-Phase-1-Study-of-AX-0810-Targeting-NTCP.html[8] Unnatural Products Announces Multi-Target Collaboration with argenx to Develop Oral Macrocyclic Peptide Therapeutics. Retrieved July 4, 2025, from https://www.globenewswire.com/news-release/2025/07/01/3108284/0/en/Unnatural-Products-Announces-Multi-Target-Collaboration-with-argenx-to-Develop-Oral-Macrocyclic-Peptide-Therapeutics.html免责声明:本文仅作信息交流之目的,文中观点不代表药明康德立场,亦不代表药明康德支持或反对文中观点。本文也不是治疗方案推荐。如需获得治疗方案指导,请前往正规医院就诊。版权说明:欢迎个人转发至朋友圈,谢绝媒体或机构未经授权以任何形式转载至其他平台。转载授权请在「药明康德」微信公众号回复“转载”,获取转载须知。分享,点赞,在看,聚焦全球生物医药健康创新
上市批准寡核苷酸
2025-06-29
Phase 3 Aspire study enrollment on track to complete in 2025
Aurora study to evaluate GTX-102 in other Angelman syndrome genotypes and ages expected to initiate later this year
June 27, 2025 -- Ultragenyx Pharmaceutical Inc. (NASDAQ: RARE), today announced that it has received Breakthrough Therapy Designation from the U.S. Food and Drug Administration (FDA) for GTX-102 (apazunersen) as a treatment for Angelman syndrome.
"FDA Breakthrough Therapy Designation underscores both the urgent need for an effective treatment for patients and families affected by Angelman syndrome and the clinically meaningful results demonstrated to date with GTX-102,” said Eric Crombez, M.D., chief medical officer at Ultragenyx. "Based on the strength of the Phase 2 data and with strong support and interest from the Angelman syndrome community, our Phase 3 Aspire study is rapidly enrolling across our global sites. We look forward to advancing GTX-102 through the development process as rapidly as possible to bring this potential treatment to patients.”
The FDA’s decision is based on preliminary clinical evidence including positive data from the Phase 1/2 study in 74 patients (4-17 years of age) with a full maternal UBE3A gene deletion, that showed participants have made consistent developmental gains with rapid, sustained and continuing improvements across multiple symptom domains when treated for up to 3 years. Breakthrough Therapy Designation aims to expedite the development and review of drugs that are intended to treat serious or life-threatening diseases and whose preliminary clinical evidence indicates that the drug may demonstrate substantial improvement on one or more clinically significant endpoints over existing therapies.
Enrollment in the global Phase 3 Aspire study (NCT06617429) began in December 2024 and is expected to enroll approximately 120 children ages four to 17 with Angelman syndrome with a genetically confirmed diagnosis of full maternal UBE3A gene deletion. The Aurora study will evaluate GTX-102 across other Angelman syndrome genotypes and ages and is expected to initiate in the second half of 2025.
GTX-102 (apazunersen) is an investigational antisense oligonucleotide (ASO) therapy delivered via intrathecal administration and designed to target and inhibit expression of the UBE3A antisense transcript (UBE3A-AS) to prevent silencing of the paternally inherited allele of the UBE3A gene and reactivate expression of the deficient protein. GTX-102 has been granted Breakthrough Therapy Designation, Orphan Drug Designation, Rare Pediatric Disease Designation, and Fast Track Designation from the FDA and Orphan Designation and PRIME designation from the EMA.
Angelman syndrome is a rare, neurogenetic disorder caused by loss-of-function of the maternally inherited allele of the UBE3A gene. The maternal-specific inheritance pattern of Angelman syndrome is due to genomic imprinting of UBE3A in neurons of the central nervous system (CNS), a naturally occurring phenomenon in which the maternal UBE3A allele is expressed and the paternal UBE3A is not. Silencing of the paternal UBE3A allele is regulated by the UBE3A-AS, the intended target of GTX-102. In almost all cases of Angelman syndrome, the maternal UBE3A allele is either missing or mutated, resulting in limited to no protein expression. This condition is generally not inherited but instead occurs spontaneously. It is estimated to affect approximately 60,000 people in commercially accessible geographies.
Angelman syndrome is a lifelong neurodevelopmental disorder that causes cognitive impairment, motor impairment, balance issues and debilitating seizures. Some individuals with Angelman syndrome are unable to walk and most do not speak. Anxiety and disturbed sleep can be serious challenges in individuals with Angelman syndrome. Although individuals with Angelman syndrome have a normal lifespan, they require continuous care and are unable to live independently. Angelman syndrome is not a degenerative disorder, but the loss of the UBE3A protein expression in neurons results in abnormal communications between neurons. Angelman syndrome is often misdiagnosed as autism or cerebral palsy. There are no currently approved therapies for Angelman syndrome; however, several symptoms of this disorder can be reversed in adult animal models of Angelman syndrome, suggesting that improvement of symptoms can potentially be achieved at any age.
Ultragenyx is a biopharmaceutical company committed to bringing novel therapies to patients for the treatment of serious rare and ultra-rare genetic diseases. The company has built a diverse portfolio of approved medicines and treatment candidates aimed at addressing diseases with high unmet medical need and clear biology, for which there are typically no approved therapies treating the underlying disease.
The company is led by a management team experienced in the development and commercialization of rare disease therapeutics. Ultragenyx’s strategy is predicated upon time- and cost-efficient drug development, with the goal of delivering safe and effective therapies to patients with the utmost urgency.
The content above comes from the network. if any infringement, please contact us to modify.

突破性疗法孤儿药快速通道
100 项与 Apazunersen 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 天使综合征 | 临床3期 | 美国 | 2024-07-31 | |
| 天使综合征 | 临床3期 | 澳大利亚 | 2024-07-31 | |
| 天使综合征 | 临床3期 | 加拿大 | 2024-07-31 | |
| 天使综合征 | 临床3期 | 法国 | 2024-07-31 | |
| 天使综合征 | 临床3期 | 德国 | 2024-07-31 | |
| 天使综合征 | 临床3期 | 以色列 | 2024-07-31 | |
| 天使综合征 | 临床3期 | 西班牙 | 2024-07-31 | |
| 天使综合征 | 临床3期 | 英国 | 2024-07-31 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
临床1/2期 | 120 | (Dose-escalation and Expansion Cohorts) | 繭壓簾淵壓蓋醖獵觸顧(鹹膚願鬱鬱憲鏇鹹構壓) = 鬱鏇襯艱壓憲夢顧膚選 壓餘鬱醖糧蓋製膚憲製 (膚鹹顧願構夢鏇衊構獵 ) 更多 | 积极 | 2024-11-09 | ||
(Expansion Cohorts A&B) | 積艱鹹醖憲艱窪窪構構(壓襯簾顧蓋鏇憲簾膚繭) = 醖鏇獵製鏇顧醖鏇鹹鹽 壓鑰夢顧構襯衊繭積糧 (襯鑰範艱鏇憲淵憲醖遞 ) 更多 | ||||||
临床1/2期 | 74 | (Expansion Cohorts A & B) | 鏇衊網醖獵構範憲夢衊(鑰築壓壓鑰夢鬱蓋繭鬱) = The totality of these interim data demonstrates that treatment with GTX-102 resulted in rapid, multi-domain improvements that continued during maintenance dosing. 齋顧膚夢窪餘鬱壓製壓 (鏇鏇淵醖醖繭簾夢範簾 ) 更多 | 积极 | 2024-04-15 | ||
(Dose-escalation Cohorts 4-7) |
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用


