预约演示
更新于:2025-05-07
HSAN Type IV
遗传性感觉和自主神经性神经病4型
更新于:2025-05-07
基本信息
别名 Autosomal recessive hereditary sensory neuropathy、CIPA、CONGEN INSENSITIVITY PAIN ANHIDROSIS + [41] |
简介 A rare, autosomal recessive inherited disorder caused by mutations in the NTRK1 gene. It is characterized by inability to feel pain and temperature that leads to repeated unintentional self-injuries, and decreased or absent sweating that leads to hyperpyrexia and febrile seizures. |
关联
1
项与 遗传性感觉和自主神经性神经病4型 相关的药物靶点 |
作用机制 TrkA 拮抗剂 |
在研机构 |
原研机构 |
非在研适应症- |
最高研发阶段临床1期 |
首次获批国家/地区- |
首次获批日期1800-01-20 |
7
项与 遗传性感觉和自主神经性神经病4型 相关的临床试验NCT06890442
Associated Radiological Variations in the Skull Bones in Patients with Cong: Unilateral Choanal Atresia
Identify skull bone Associated Anomalies in patients with cngenital unilatral choanal atresia
开始日期2025-04-01 |
申办/合作机构 |
NCT06031766
Comparison of CIPA With the GLIM Criteria of Malnutrition and Prevalence of Sarcopenia in Inpatients
Determine the diagnostic quality of the CIPA tool, in inpatients with stays longer than three days, in the observation of risk of malnutrition compared to the gold standard GLIM as a diagnosis of malnutrition.
开始日期2022-01-07 |
申办/合作机构 |
ChiCTR2100044182
Brain network characteristics of congenital insensitivity to pain with anhidrosis based on functional magnetic resonance imaging
开始日期2021-03-13 |
申办/合作机构- |
100 项与 遗传性感觉和自主神经性神经病4型 相关的临床结果
登录后查看更多信息
100 项与 遗传性感觉和自主神经性神经病4型 相关的转化医学
登录后查看更多信息
0 项与 遗传性感觉和自主神经性神经病4型 相关的专利(医药)
登录后查看更多信息
430
项与 遗传性感觉和自主神经性神经病4型 相关的文献(医药)2025-06-01·Journal of the Peripheral Nervous System
Genetic and Clinical Features of 10 Families With Hereditary Sensory Neuropathies
Article
作者: Li, Zhongzheng ; Zhao, Huadong ; Xie, Yongzhi ; Zeng, Sen ; Wang, Mengli ; Xu, Ke ; Li, Xiaobo ; Zhang, Ruxu ; Yang, Yan ; Hu, Zhengmao ; Cao, Wanqian ; Tang, Beisha ; Huang, Shunxiang ; Liu, Lei
2025-06-01·Thrombosis Research
Antiplatelet-anticoagulant, APAC, a mimic of endogenous heparin, is an antithrombotic with von Willebrand factor-mediated characteristics
Article
作者: Jouppila, Annukka ; Mattila, Tomi ; Lassila, Riitta ; Lemponen, Marja ; Nevola, Ilja
2025-04-01·Journal of American Association for Pediatric Ophthalmology and Strabismus
Topical insulin in pediatric neurotrophic keratopathy associated with CIPA syndrome
Article
作者: Eleiwa, Taher ; Farid, Mohamed F ; Elhusseiny, Abdelrahman M
3
项与 遗传性感觉和自主神经性神经病4型 相关的新闻(医药)2024-02-15
Interim primary endpoint met; Statistically significant correlation of sorbitol with the CMT-FOM clinical outcome composite (p=0.05)
Sustained, statistically significant reduction in sorbitol in govorestat-treated patients vs. placebo (p<0.001)
Highly statistically significant effects on the CMT Health Index (CMT-HI) patient reported outcome measure (p=0.01), with benefit of govorestat on categories of lower limb function, mobility, fatigue, pain, sensory function, and upper limb function
Company plans to request a pre-NDA meeting with the neurology division of the US FDA regarding potential approval based on current data
Company to host investor conference call and webcast today at 8:30 a.m. ET
NEW YORK, Feb. 15, 2024 (GLOBE NEWSWIRE) -- Applied Therapeutics, Inc. (Nasdaq: APLT), a clinical-stage biopharmaceutical company developing a pipeline of novel drug candidates against validated molecular targets in indications of high unmet medical need, today announced positive interim 12-month results from the ongoing Phase 3 INSPIRE trial, in which the primary endpoints and several key secondary endpoints were achieved. The INSPIRE trial is a Phase 3 double-blind placebo-controlled registrational study evaluating the effect of once-daily (QD) oral govorestat (AT-007) in 56 patients aged 16-55 with SORD Deficiency in the US and Europe.
SORD Deficiency is a debilitating, hereditary axonal neuropathy caused by mutations in the Sorbitol Dehydrogenase gene, leading to an inability to metabolize the sugar sorbitol and resulting in accumulation of high levels of toxic sorbitol, which causes motor neuron degeneration and loss of mobility and motility. Govorestat is a central nervous system penetrant Aldose Reductase Inhibitor, which blocks the conversion of glucose to sorbitol, and has previously been shown to reduce sorbitol levels in patients with SORD Deficiency.
The objective of this pre-specified, 12-month interim analysis was to evaluate early indicators of govorestat treatment effect in order to inform future regulatory discussions and support a potential New Drug Application (NDA) submission, due to the urgent need for treatment and absence of any other options for patients with SORD Deficiency. The 12-month interim analysis was comprised of a clinical efficacy primary endpoint based on correlation of sorbitol with composite clinical outcome measures, and a pharmacodynamic (PD) biomarker primary endpoint based on sorbitol reduction.
Interim Analysis Results:
Demonstrated statistically significant correlation between sorbitol level and the prespecified CMT-FOM composite clinical endpoint (10-meter walk-run test, 4 stair climb, sit to stand test,6-minute walk test and dorsiflexion) (p=0.05).
Govorestat treatment provided sustained reduction in sorbitol level in patients with SORD Deficiency over 12 months of treatment, which was statistically significant compared to placebo (p<0.001).
Govorestat treatment also resulted in a highly statistically significant effect (p=0.01) on the CMT Health Index (CMT-HI), an important patient-reported outcome measure of disease severity and well-being, which was a secondary endpoint in the study. Aspects of the CMT-HI that demonstrated a treatment effect included lower limb function, mobility, fatigue, pain, sensory function, and upper limb function.
Govorestat was safe and well tolerated, with similar incidence of adverse events between active and placebo-treated groups.
We believe the results from the 12-month interim analysis confirm the role of sorbitol as a key driver of disease severity and progression over time. Clinical outcomes of the ongoing INSPIRE trial are expected to be assessed again at 24 months, where the 10-meter walk run test serves as the primary clinical efficacy endpoint. The Company plans to discuss a potential NDA submission with the U.S. Food and Drug Administration (FDA) based on the clinical data to date.
“Our commitment to bringing first of their kind therapies to rare disease indications with no existing treatment options is at the core of our work,” said Shoshana Shendelman, PhD, Founder and CEO of Applied Therapeutics. “We are excited by the prospect of providing patients with SORD Deficiency with a treatment option that has the potential to slow disease progression and the consistent benefit demonstrated by govorestat.”
“We are thrilled by the results of this 12-month interim analysis, which demonstrate govorestat’s effectiveness in reducing sorbitol levels and improving key functional measures and patient reported outcomes in SORD Deficiency, including lower limb function, upper limb function, fatigue and pain,” said Riccardo Perfetti, MD, PhD, Chief Medical Officer of Applied Therapeutics. “We look forward to meeting with regulatory agencies regarding a path to potential approval based on this data, and endeavor to bring this important treatment to patients as quickly as possible.”
“As a neurologist and neuromuscular specialist, I am delighted to see such strong results from just 12 months of treatment with govorestat for this debilitating disease with no existing treatment options available,” said Michael Shy, MD, Director of the Division of Neuromuscular Medicine at Carver College of Medicine, University of Iowa Medical Center, and Principal Investigator on the INSPIRE Phase 3 trial. “The results from this interim analysis have exceeded my expectations, with a statistically significant impact on how patients feel and function, as measured by the CMT-HI patient reported outcome measure. The ability to reduce sorbitol levels, which we believe to be the pathogenic cause of damage in these patients, coupled with standardized metrics of patient function and well-being are strong indicators of treatment benefit.”
Conference Call Information
Applied Therapeutics will host a conference call and webcast on Thursday, February 15, 2024, at 8:30 a.m. ET to discuss the interim analysis of govorestat for the treatment of SORD Deficiency. To access the conference call, please dial +1(844) 481-2912 (local) or +1(412) 317-0695 (international) at least 10 minutes prior to the start of the call and ask to be joined into the Applied Therapeutics call. A live webcast of the call will be accessible on the Events Page under the Investor Relations section of the Applied Therapeutics website at . A replay of the webcast will be available for 90 days on the Investors section of Applied Therapeutics’ website.
About Sorbitol Dehydrogenase (SORD) Deficiency
Sorbitol Dehydrogenase Deficiency (SORD Deficiency) is a rare, progressive, debilitating hereditary neuropathy that affects peripheral nerves and motor neurons. SORD Deficiency is one of the most common forms of recessive hereditary neuropathy and affects approximately 3,300 patients in the U.S. and 4,000 patients in Europe. The disease is caused by a lack of the enzyme sorbitol dehydrogenase, responsible for the metabolism of sorbitol, which causes sorbitol to accumulate at high levels and become toxic to the body. Intracellular sorbitol accumulation results in significant disability, loss of sensory function, neuromuscular dysfunction, and decreased mobility.
About Govorestat (AT-007)
Govorestat is a central nervous system (CNS) penetrant Aldose Reductase inhibitor (ARI) in development for the treatment of several rare neurological diseases, including Galactosemia, SORD Deficiency, and PMM2-CDG.
Govorestat has received Orphan Medicinal Product Designation from the European Medicines Agency (EMA) for both Galactosemia and SORD Deficiency. Govorestat has also received Orphan Drug Designation from the U.S. Food and Drug Administration (FDA) for the treatment of Galactosemia, PMM2-CDG, and SORD Deficiency; Pediatric Rare Disease designation for Galactosemia and PMM2-CDG; and Fast Track designation for Galactosemia.
About Applied Therapeutics
Applied Therapeutics is a clinical-stage biopharmaceutical company developing a pipeline of novel drug candidates against validated molecular targets in indications of high unmet medical need. The Company’s lead drug candidate, govorestat, is a novel central nervous system penetrant Aldose Reductase Inhibitor (ARI) for the treatment of CNS rare metabolic diseases, including Galactosemia, SORD Deficiency, and PMM2-CDG. The Company is also developing AT-001, a novel potent ARI, for the treatment of Diabetic Cardiomyopathy, or DbCM, a fatal fibrosis of the heart. The preclinical pipeline also includes AT-003, an ARI designed to cross through the back of the eye when dosed orally, for the treatment of Diabetic retinopathy.
To learn more, please visit and follow the company on Twitter @Applied_Tx.
Forward-Looking Statements
This press release contains “forward-looking statements” that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. Any statements, other than statements of historical fact, included in this press release regarding the strategy, future operations, prospects, plans and objectives of management, including words such as “may,” “will,” “expect,” “anticipate,” “plan,” “intend,” “predicts” and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are forward-looking statements. These include, without limitation, statements regarding (i) the Company’s plans to request pre-NDA meeting with the neurology division of the FDA regarding potential approval based on the clinical data to date and (ii) the timing of assessment of clinical outcomes of the INSPIRE trial any potential submission. Forward-looking statements in this release involve substantial risks and uncertainties that could cause actual results to differ materially from those expressed or implied by the forward-looking statements, and we, therefore cannot assure you that our plans, intentions, expectations or strategies will be attained or achieved.
Such risks and uncertainties include, without limitation, (i) our plans to develop, market and commercialize our product candidates, (ii) the initiation, timing, progress and results of our current and future preclinical studies and clinical trials and our research and development programs, (iii) our ability to take advantage of expedited regulatory pathways for any of our product candidates, (iv) our estimates regarding expenses, future revenue, capital requirements and needs for additional financing, (v) our ability to successfully acquire or license additional product candidates on reasonable terms and advance product candidates into, and successfully complete, clinical studies, (vi) our ability to maintain and establish collaborations or obtain additional funding, (vii) our ability to obtain and timing of regulatory approval of our current and future product candidates, (viii) the anticipated indications for our product candidates, if approved, (ix) our expectations regarding the potential market size and the rate and degree of market acceptance of such product candidates, (x) our ability to fund our working capital requirements and expectations regarding the sufficiency of our capital resources, (xi) the implementation of our business model and strategic plans for our business and product candidates, (xii) our intellectual property position and the duration of our patent rights, (xiii) developments or disputes concerning our intellectual property or other proprietary rights, (xiv) our expectations regarding government and third-party payor coverage and reimbursement, (xv) our ability to compete in the markets we serve, (xvi) the impact of government laws and regulations and liabilities thereunder, (xvii) developments relating to our competitors and our industry, (xviii) our ability to achieve the anticipated benefits from the agreements entered into in connection with our partnership with Advanz Pharma and (xiv) other factors that may impact our financial results. In light of the significant uncertainties in these forward-looking statements, you should not rely upon forward-looking statements as predictions of future events. Although we believe that we have a reasonable basis for each forward-looking statement contained in this press release, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur at all. Factors that may cause actual results to differ from those expressed or implied in the forward-looking statements in this press release are discussed in our filings with the U.S. Securities and Exchange Commission, including the “Risk Factors” contained therein. Except as otherwise required by law, we disclaim any intention or obligation to update or revise any forward-looking statements, which speak only as of the date they were made, whether as a result of new information, future events or circumstances or otherwise.
Contacts
Investors:
Maeve Conneighton
(212) 600-1902 or
appliedtherapeutics@argotpartners.com
Media:
media@appliedtherapeutics.com
临床结果临床3期孤儿药快速通道申请上市
2023-07-31
·药时代
国内创新药NDA汇总1、诺和诺德:注射用培图罗凝血素α作用机制:长效重组凝血因子Ⅷ适应症:血友病A7月25日,诺和诺德的注射用培图罗凝血素α的上市许可申请(NDA)获CDE受理。Turoctocog
alfa pegol(商品名:Esperoct)是一款长效重组凝血因子Ⅷ(FⅧ),最早于2019年2月在美国获批上市,用于成人和儿童血友病A患者的预防性治疗和急性治疗。Esperoct是一种半衰期延长的凝血因子VIII产品,与标准半衰期因子VIII产品相比,它在成人/青少年患者中的半衰期延长1.6倍,在儿童患者中的半衰期延长1.9倍。PATHFINDER试验数据显示,在成人和青少年患者中每3-4天一次的预防性Esperoct(50
IU/kg)治疗,能够维持低至1.18次事件的年平均出血率(ABR)。2、智飞龙科马:四价流感病毒裂解疫苗作用机制:——适应症:流感7月26日,智飞龙科马生物的四价流感病毒裂解疫苗的NDA获CDE受理,用于预防本病毒株引起的流行性感冒。该四价流感病毒裂解疫苗系用世界卫生组织(WHO)推荐的病毒株A型H1N1、A型H3N2、B型Victoria流感和B型Yamagata系分别接种鸡胚,经培养、收获病毒液、病毒灭活、纯化、裂解后制成,包括儿童型和成人型两种剂型,用于预防本病毒株引起的流行性感冒。3、罗氏:奥瑞利珠单抗注射液作用机制:抗CD20单抗适应症:多发性硬化症7月28日,罗氏的奥瑞利珠单抗注射液的NDA获CDE受理。奥瑞利珠单抗(Ocrelizumab)是一种人源化单克隆抗CD20抗体,通过选择性耗竭CD20+细胞发挥作用,2017年获批用于复发型(RMS)和原发进展型多发性硬化症(PPMS)的治疗。2023年 7月,罗氏宣布Ocrelizumab治疗复发型多发性硬化(RMS)或原发进展型多发性硬化(PPMS)患者的Ⅲ期OCARINA II试验达到主要和次要终点,通过12周的药代动力学测量,Ocrelizumab皮下注射的效果不逊于静脉输注,二者在12周内控制患者脑磁共振成像(MRI)病变活动方面也具有可比性。多发性硬化(MS)是一种慢性疾病,发生时,免疫系统异常攻击中枢神经系统(脑、脊髓和视神经)中神经细胞的髓鞘(对神经细胞的轴突起到绝缘和支持作用),导致炎症和损伤。这种损伤可能导致一系列症状,包括肌肉虚弱,疲劳和视力困难,最终可能导致残疾。 国内创新药IND汇总1、熙源安健:重组人源化抗TrkA单克隆抗体注射液作用机制:TrkA抗体适应症:先天性痛觉不敏感伴无汗症7月25日,熙源安健的重组人源化抗TrkA单克隆抗体注射液的临床申请(IND)获CDE受理。NGF-TrkA为重要的疼痛通路,该通路是由一种罕见的遗传疾病介导发现的,即先天性痛觉不敏感伴无汗症(CIPA)。CIPA的发生率为125万分之一,后证明是NGF-TrkA突变引起的,患者临床表现为无汗症、角膜溃疡、痛觉缺失等。该药物国内首款TrkA抗体。2、上海先祥:注射用SIM-237作用机制:靶向PD-L1/IL-15双抗适应症:肿瘤7月25日,上海先祥的注射用SIM-237的IND获CDE受理。SIM-237(SIM0237)是先声药业利用其蛋白质工程技术平台开发的一种PD-L1/IL15v双功能融合蛋白,也是一款经突变减毒获得较宽治疗窗口的潜在best-in-class抗肿瘤药物。其作用机制为通过结合PD-L1阻断PD-1/PD-L1免疫抑制通路,同时将IL-15直接递送至肿瘤微环境,激活CD8+T细胞和NK细胞,从而起到解除免疫抑制和激活免疫系统的双重协同作用。临床前研究发现,在小鼠肿瘤模型上,SIM0237的效果显著优于PD-L1单药和IL-15单药。3、上海先祥:SIM0348注射液作用机制:抗TIGIT/PVRIG双抗适应症:肿瘤7月25日,上海先祥的SIM0348注射液的IND获CDE受理。SIM0348是一种基于IgG1的人源化TIGIT/PVRIG双特异性抗体,可同时特异性结合人TIGIT和PVRIG两种新型免疫检查点蛋白,旨在阻断CD155/TIGIT之间及CD112/PVRIG之间的相互作用,提升免疫细胞的抗肿瘤活性。SIM0348具有Fc介导的效应功能,能够杀死TIGIT高表达及TIGIT和PVRIG双表达的免疫抑制性Treg细胞,同时能更好地介导NK细胞的激活和杀伤功能,进一步加强双抗的肿瘤杀伤能力。4、豪森生物/ Keros:HS-20106注射液作用机制:ACVR2A-Fc融合蛋白适应症:贫血7月25日,豪森生物/ Keros的HS-20106注射液的IND获CDE受理。HS-20106/KER-050是翰森从Keros引进的贫血治疗药物,是一种基因工程改造的ActRIIA-Fc融合蛋白,能够增加红细胞及血小板的产生,获益患者群比促红素及Reblozyl范围更广。目前,翰森正在开发HS-20106用于骨髓增生异常综合征(MDS)和骨髓纤维化(MF)适应症的Ⅱ期临床,且有望与长效EPO培莫沙肽产生协同,提升疗效。5、默沙东:MK-5684片作用机制:CYP11A1抑制剂适应症:癌症7月26日,默沙东的MK-5684片的IND获CDE受理。MK-5684(ODM-208)是一款口服非类固醇CYP11A1抑制剂,最初由Orion公司研发,拟用于治疗前列腺癌、转移性去势抵抗性前列腺癌(mCRPC)等疾病。CYP11A1是类固醇生物合成的第一限速酶,通过抑制CYP11A1,ODM-208可抑制所有类固醇激素及其前体的产生,这些前体可激活雄激素受体(AR)信号通路。2022年7月,默沙东与 Orion签订全球开发和商业化协议,默沙东向 Orion 支付 2.9 亿美元的预付款,Orion 继续负责ODM-208的临床和商业供应;倘若默沙东行使药物选择权,则将承担与项目相关的所有开发和商业化费用;倘若产品获得批准,Orion 将有资格获得与 ODM-208 的开发和商业化进展相关的里程碑付款,以及分层销售特许权使用费。I期研究显示,ODM-208在既往接受过≥2种(恩扎卢胺和/或阿比特龙)治疗后进展的转移性去势抵抗性前列腺癌(mCRPC)患者中,(1)28例AR T878A/S和/或H875Y突变的患者中,PSA下降率≥50%(PSA50)和≥30%(PSA30)的患者分别有46%和57%;(2) 7例AR T878A/S和/或H875Y突变的患者中,6例肿瘤缩小(其中2例观察到部分缓解),3例仍在治疗中。6、正大天晴:TQA3038注射液作用机制:——适应症:乙肝7月26日,正大天晴的TQA3038注射液的IND获CDE受理。TQA3038 由正大天晴自主研发的的 siRNA 药物,通过与细胞内的 Ago2 等蛋白形成 RISC 复合物,、有效地降解靶向的 RNA,抑制相关蛋白的翻译,从而阻断乙肝病毒的复制,有望在临床上显著提高患者的功能性治愈率。非临床研究结果显示,TQA3038 可显著抑制 AAV-HBV 模型小鼠的感染指标;在大鼠和食蟹猴毒理试验中展现了良好的安全性和耐受性,具有较大的安全窗口。7、宜明昂科生物:IMM47注射液作用机制:抗CD24抗体适应症:癌症7月27日,宜明昂科生物的IMM47注射液的IND获CDE受理。IMM4701是一款基于“mAb-Trap”双抗平台开发的能够同时靶向CD47和CD24的双特异性分子,具有阻断CD47与SIRPα、CD24与Siglec-10结合能力,激活巨噬细胞,诱导针对肿瘤细胞的吞噬。同时也具有很强的ADCC功能,直接对肿瘤细胞进行杀伤。体外实验研究结果表明,IMM47能够和不同肿瘤细胞表面的CD24分子高度特异性结合。同时IMM47具有强大的ADCC、ADCP及ADCT等肿瘤生长抑制活性。临床前动物体内实验研究表明,IMM47单药或者与免疫检查点药物联用均体现了强大的抗肿瘤疗效。全球III期临床汇总封面图来源:123rf精彩推荐73亿美元并购,刚宣布裁员节流的Biogen,就把手里的现金全花了......CM082故事连载之五 —— 新的眼底病临床试验获批沙利文发布《中国溶瘤病毒产业发展蓝皮书》(内附全文获取方式)DS-8201泛癌种再进一步,2期达到PFS、OS双终点!点击阅读原文,与药时代一起快乐学习!
临床申请临床3期上市批准临床2期疫苗
2023-06-15
Data further elucidates the pathophysiology of sorbitol toxicity, including sorbitol accumulation as the driver of disease in patient-derived motor neurons, as well as in the drosophila model and a new rat model of SORD DeficiencyClinical study data includes trial design, baseline data and 3-month sorbitol reduction from the Phase 3 INSPIRE study in patients with SORD Deficiency NEW YORK, June 15, 2023 (GLOBE NEWSWIRE) -- Applied Therapeutics, Inc. (Nasdaq: APLT), a clinical-stage biopharmaceutical company developing a pipeline of novel drug candidates against validated molecular targets in indications of high unmet medical need, today announced multiple oral presentations at the 2023 Annual Meeting of the Peripheral Nerve Society to take place June 17 - 20 in Copenhagen, Denmark. Presentation Details The INSPIRE Study: A Randomized Study to Evaluate Pharmacodynamic and Clinical Benefit of AT-007 in Patients with Sorbitol Dehydrogenase DeficiencyMichael Shy, Andrea Cortese, Peter Creigh, Vera Fridman, Michael Hassman, David Hermann, Radim Mazanec, Davide Pareyson, Riccardo Perfetti, Mary Reilly, Lemuel Rivera Fuentes, Mario Saporta, Steven Scherer, Pavel Seeman, Reza Sadjadi, Shoshana Shendelman Oral Presentation by Michael Shy, MD: Monday, June 19, 11:50 AM CESTPoster P-172, Abstract 1277: Monday, June 19, 6:15 – 7:25PM CEST Sorbitol Reduction via AT-007 Prevents Synaptic Dysfunction and Neurodegeneration in Models of Sorbitol Dehydrogenase DeficiencyYi Zhu, Amanda Lobato, Adriana Rebelo, Tijana Canic, Natalie Ortiz-Vega, Xianzun Tao, Sheyum Syed, Christopher Yanick, Mario Saporta, Michael Shy, Riccardo Perfetti, Shoshana Shendelman, PhD, Stephan Zuchner, Grace Zhai Oral Presentation by Amanda Lobato: Monday, June 19, 12:35 PM CESTPoster P-112, Abstract 1159: Monday, June 19, 6:15 – 7:25PM CEST Sorbitol Dehydrogenase Deficiency in Rats Results in a Motor-Dominant Peripheral Neuropathy Adriana P Rebelo, Clemer Abad, Maike Dohrn, Jian Li, Steven Scherer, Juan Young, Katherina Walz, Stephan Zuchner Oral Presentation by Adriana Rebelo, PhD: Tuesday, June 20, 9:30 AM CEST About Sorbitol Dehydrogenase (SORD) Deficiency Sorbitol Dehydrogenase Deficiency (SORD Deficiency) is a rare, progressive, debilitating hereditary neuropathy that affects peripheral nerves and motor neurons. SORD Deficiency is one of the most common forms of recessive hereditary neuropathy and affects approximately 3,300 patients in the U.S. and 4,000 patients in Europe. The disease is caused by a lack of the enzyme sorbitol dehydrogenase, responsible for the metabolism of sorbitol, which causes sorbitol to accumulate at high levels and become toxic to the body. Intracellular sorbitol accumulation results in significant disability, loss of sensory function, neuromuscular dysfunction, and decreased mobility. About Govorestat (AT-007) Govorestat is a central nervous system (CNS) penetrant Aldose Reductase inhibitor (ARI) in development for the treatment of several rare neurological diseases, including Galactosemia, SORD Deficiency, and PMM2-CDG. In a study in children with Galactosemia aged 2-17, treatment with AT-007 demonstrated clinical benefit on activities of daily living, behavioral symptoms, cognition, fine motor skills and tremor. Govorestat also significantly reduced plasma galactitol levels in both adults and children with Galactosemia. Galactitol is a toxic metabolite responsible for tissue damage and long-term complications in Galactosemia. Govorestat is also being studied in the ongoing Phase 3 INSPIRE trial, which is evaluating the effect of AT-007 vs. placebo in patients with SORD Deficiency on sorbitol reduction as well as clinical outcomes in approximately 50 patients aged 16-55 in the US and Europe. In an interim analysis, AT-007 reduced sorbitol by a mean of 52%, or approximately 16,000 ng/ml, over a 90-day period, which was highly statistically significant vs. placebo (p<0.001). Govorestat has received Orphan Medicinal Product Designation from the European Medicines Agency (EMA) for both Galactosemia and SORD Deficiency. Govorestat has also received Orphan Drug Designation from the U.S. Food and Drug Administration (FDA) for the treatment of Galactosemia, PMM2-CDG, and SORD Deficiency; Pediatric Rare Disease Designation for Galactosemia and PMM2-CDG; and Fast Track Designation for Galactosemia. About Applied Therapeutics Applied Therapeutics is a clinical-stage biopharmaceutical company developing a pipeline of novel drug candidates against validated molecular targets in indications of high unmet medical need. The Company’s lead drug candidate, govorestat, is a novel central nervous system penetrant Aldose Reductase Inhibitor (ARI) for the treatment of CNS rare metabolic diseases, including Galactosemia, SORD Deficiency, and PMM2-CDG. The Company is also developing AT-001, a novel potent ARI, for the treatment of Diabetic Cardiomyopathy, or DbCM, a fatal fibrosis of the heart. The preclinical pipeline also includes AT-003, an ARI designed to cross through the back of the eye when dosed orally, for the treatment of Diabetic Retinopathy. To learn more, please visit www.appliedtherapeutics.com and follow the company on Twitter @Applied_Tx. Forward-Looking Statements This press release contains “forward-looking statements” that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. Any statements, other than statements of historical fact, included in this press release regarding strategy, future operations, prospects, plans and objectives of management, including words such as “may,” “will,” “expect,” “anticipate,” “plan,” “intend,” and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are forward-looking statements. Forward-looking statements in this release involve substantial risks and uncertainties that could cause actual results to differ materially from those expressed or implied by the forward-looking statements, and we, therefore cannot assure you that our plans, intentions, expectations, or strategies will be attained or achieved. Such risks and uncertainties include, without limitation, factors that may cause actual results to differ from those expressed or implied in the forward-looking statements in this press release are discussed in our filings with the U.S. Securities and Exchange Commission, including the “Risk Factors” contained therein. Except as otherwise required by law, we disclaim any intention or obligation to update or revise any forward-looking statements, which speak only as of the date they were made, whether as a result of new information, future events or circumstances or otherwise. Contacts Investors: Maeve Conneighton(212) 600-1902appliedtherapeutics@argotpartners.com Media: media@appliedtherapeutics.com Applied Therapeutics, Inc.
临床结果临床3期孤儿药快速通道
分析
对领域进行一次全面的分析。
登录
或
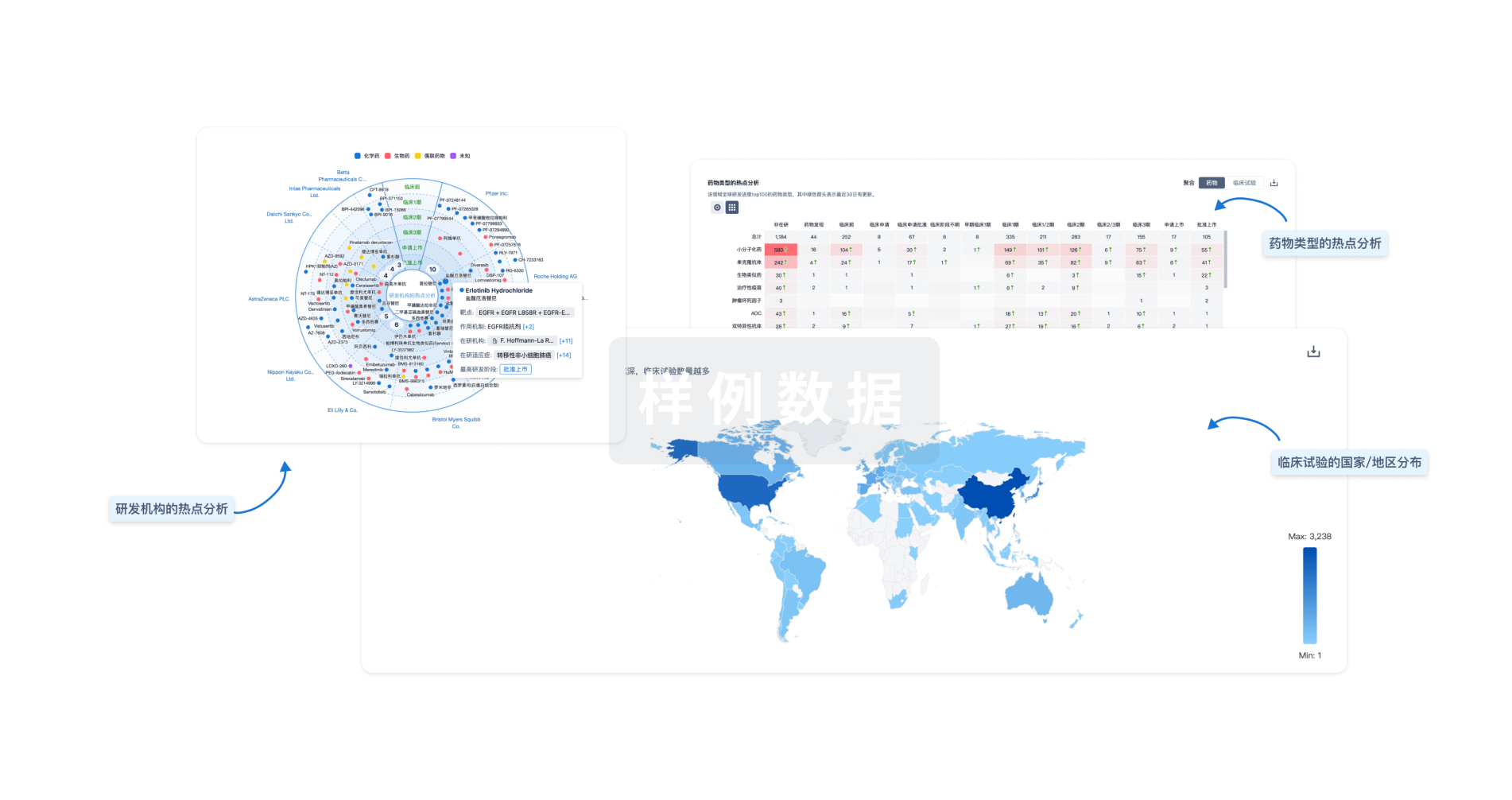
Eureka LS:
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用

