预约演示
更新于:2025-05-07
Lipase x CLPS
更新于:2025-05-07
关联
1
项与 Lipase x CLPS 相关的药物作用机制 CLPS抑制剂 [+1] |
在研机构- |
在研适应症- |
非在研适应症 |
最高研发阶段无进展 |
首次获批国家/地区- |
首次获批日期1800-01-20 |
100 项与 Lipase x CLPS 相关的临床结果
登录后查看更多信息
100 项与 Lipase x CLPS 相关的转化医学
登录后查看更多信息
0 项与 Lipase x CLPS 相关的专利(医药)
登录后查看更多信息
364
项与 Lipase x CLPS 相关的文献(医药)2025-03-01·Pharmacological Research
Preclinical development of a standardized extract of Ilex paraguariensis A.St.-Hil for the treatment of obesity and metabolic syndrome
Article
作者: Siqueira Júnior, Jarbas Mota ; Marcon, Rodrigo ; Macedo Júnior, Sergio José ; Vilela, Fabiana Cardoso ; Heller, Melina ; Rocha, Ruth Fernandes ; Andrade, Edineia Lemos de ; Marques, Naiani Ferreira ; Tolouei, Sara E L ; Calixto, João B ; Santos, Adara Áurea Dos ; Freitas, Cristina Setim ; Fadanni, Guilherme Pasetto
2024-12-11·Journal of Agricultural and Food Chemistry
AlphaFold 3.0 Prediction Reveals Stronger Interaction between Oleic Acid and Colipase than Palmitic Acid
Article
作者: Ye, Zhan ; Zheng, Liyou ; Zhou, Xiaoting ; Zhang, Tao
2023-10-01·EFSA Journal
Appethyl® and reduction of body weight: evaluation of a health claim pursuant to Article 13(5) of Regulation (EC) No 1924/2006
Article
作者: Vinceti, Marco ; Turck, Dominique ; Maciuk, Alexandre ; Bohn, Torsten ; Naska, Androniki ; Bresson, Jean‐Louis ; Siani, Alfonso ; Hirsch‐Ernst, Karen Ildico ; McArdle, Harry J ; Bresson, Jean-Louis ; Knutsen, Helle Katrine ; Castenmiller, Jacqueline ; Hirsch-Ernst, Karen Ildico ; Pentieva, Kristina ; Thies, Frank ; Mangelsdorf, Inge ; Fiolet, Thibault ; De Henauw, Stefaan ; Tsabouri, Sophia
分析
对领域进行一次全面的分析。
登录
或
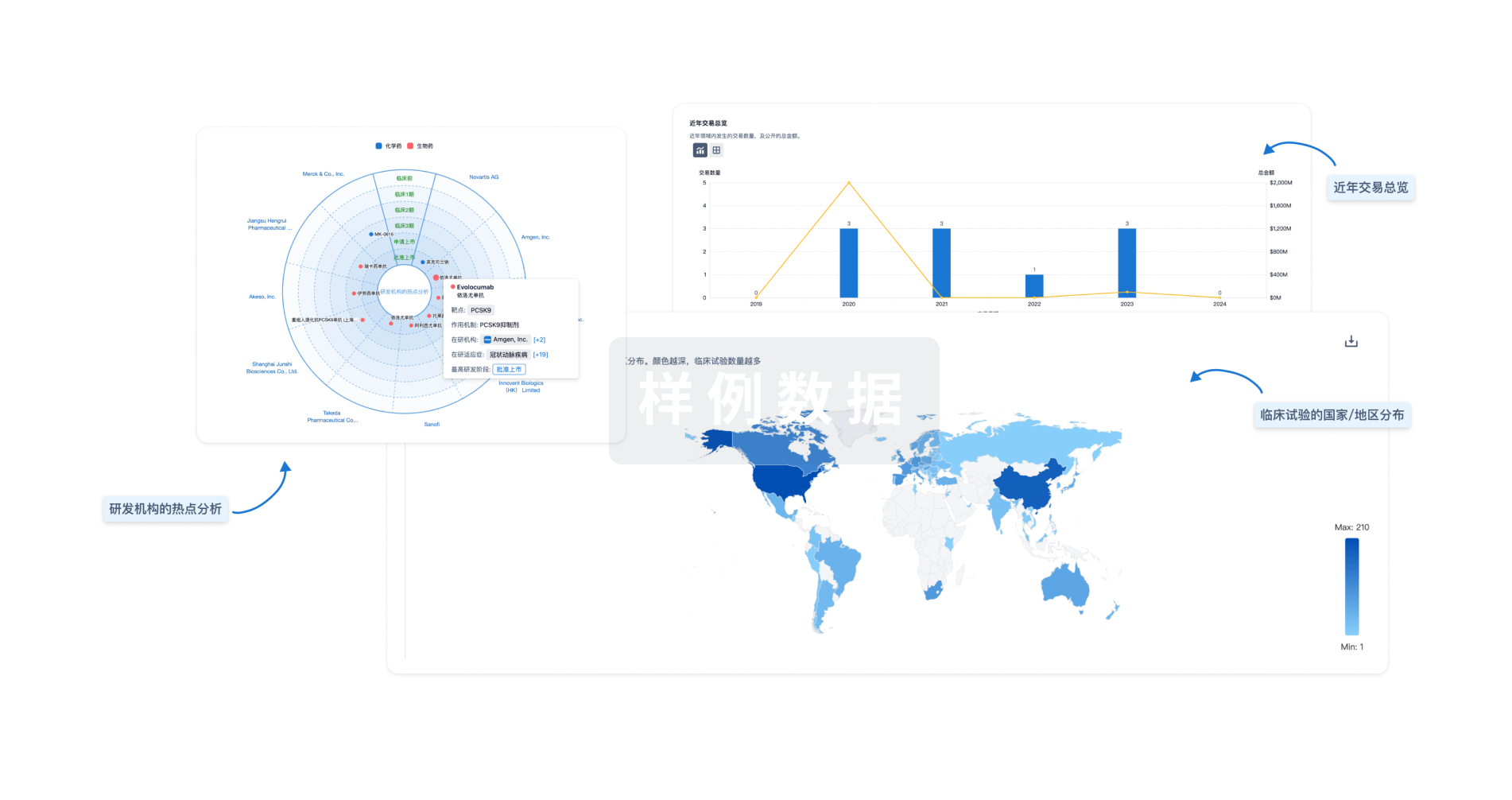
生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用