更新于:2024-11-01
PDE5A x PRKAB1
更新于:2024-11-01
关联
1
项与 PDE5A x PRKAB1 相关的药物作用机制 PDE5A抑制剂 [+1] |
在研适应症 |
非在研适应症 |
最高研发阶段临床2期 |
首次获批国家/地区- |
首次获批日期1800-01-20 |
2
项与 PDE5A x PRKAB1 相关的临床试验A Randomized, Double-blind, Placebo-controlled Study of Effects of Two Fixed-dose Leucine-sildenafil Combinations (NS-0300) or Two Fixed-dose Leucine-sildenafil-metformin Combinations (NS-0200) Versus Placebo on Body Weight in Obesity
This is a 24-week study to evaluate the effects of two fixed-dose combinations of leucine and sildenafil or two fixed-dose combinations of leucine, sildenafil and metformin compared to placebo. The primary objective of this study is to evaluate the percentage change in body weight in subjects from Baseline/Visit 2 (Day 1) to Study Termination/Visit 8 (Day 168/Week 24).
开始日期2018-01-08 |
申办/合作机构 |
A Randomized, Blinded, Placebo-Controlled Study To Evaluate The Effect Fixed-Dose Leucine, Metformin, Sildenafil Combinations(NS-0200) Versus Placebo On Hepatic Fat Assessed By MRI In Non Alcoholic Fatty Liver Disease Patients
The goal of this study is to determine if NS-0200 can reduce the amount of liver fat in patients diagnosed with non-alcoholic fatty liver disease (NAFLD). This study will compare two doses of NS-0200 to placebo in NAFLD patients.
开始日期2015-11-19 |
申办/合作机构 |
100 项与 PDE5A x PRKAB1 相关的临床结果
登录后查看更多信息
100 项与 PDE5A x PRKAB1 相关的转化医学
登录后查看更多信息
0 项与 PDE5A x PRKAB1 相关的专利(医药)
登录后查看更多信息
分析
对领域进行一次全面的分析。
登录
或
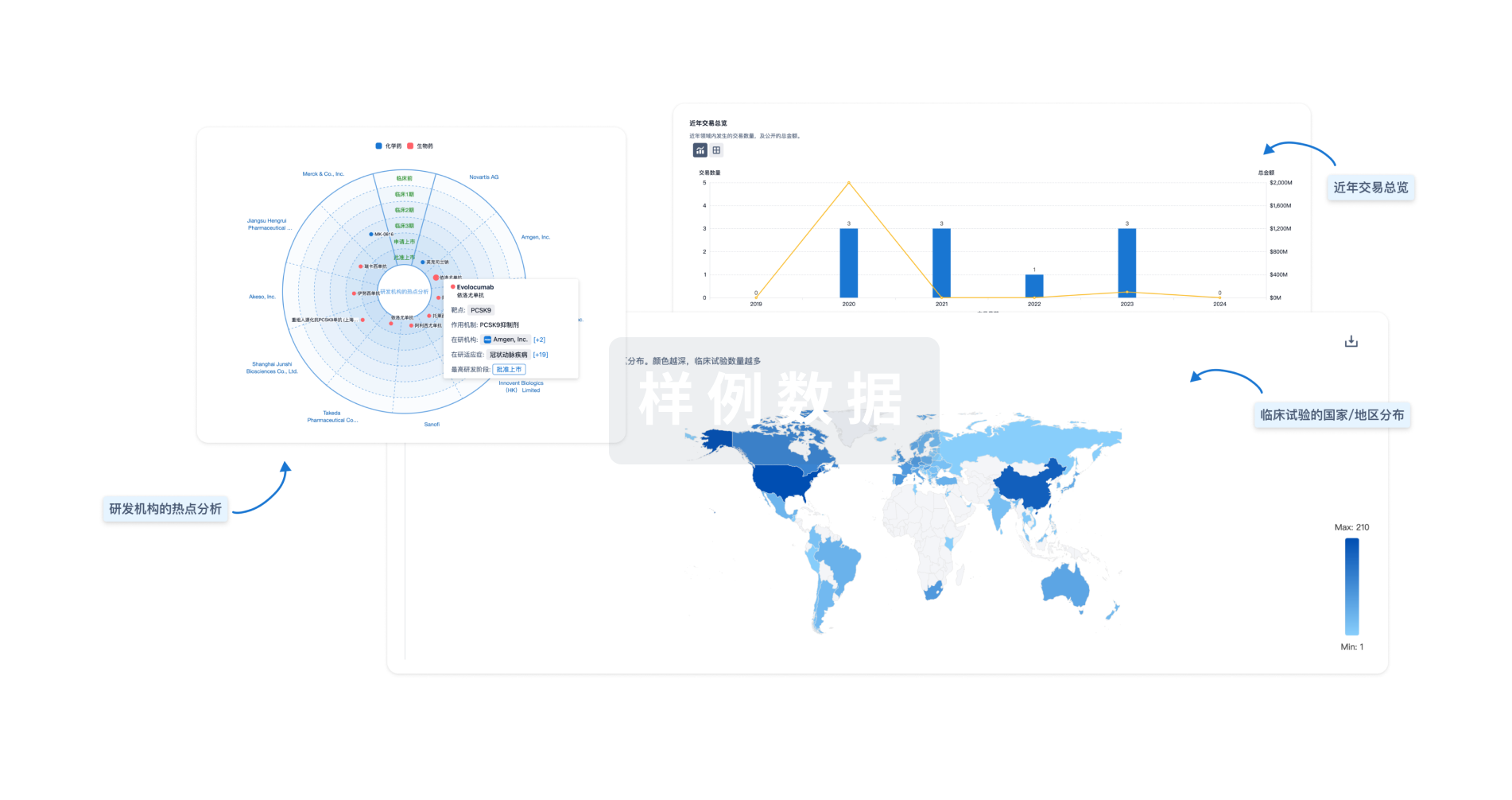
标准版
¥16800
元/账号/年
新药情报库 | 省钱又好用!
立即使用
来和芽仔聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用
