预约演示
更新于:2025-05-07
醇溶蛋白
更新于:2025-05-07
基本信息
别名- |
简介- |
关联
2
项与 醇溶蛋白 相关的药物靶点 |
作用机制 醇溶蛋白刺激剂 [+2] |
在研机构 |
在研适应症 |
非在研适应症- |
最高研发阶段临床1期 |
首次获批国家/地区- |
首次获批日期1800-01-20 |
靶点 |
作用机制 醇溶蛋白 抑制剂 |
在研机构- |
在研适应症- |
非在研适应症 |
最高研发阶段无进展 |
首次获批国家/地区- |
首次获批日期1800-01-20 |
100 项与 醇溶蛋白 相关的临床结果
登录后查看更多信息
100 项与 醇溶蛋白 相关的转化医学
登录后查看更多信息
0 项与 醇溶蛋白 相关的专利(医药)
登录后查看更多信息
698
项与 醇溶蛋白 相关的文献(医药)2025-06-01·Talanta
Electrochemical tracking of gluten in marketed foods by using a recombinant antibody fragment based-platform
Article
作者: Martín, Rosario ; Garcia-Calvo, Eduardo ; Campuzano, Susana ; Rodríguez, Santiago ; García, Teresa ; Ruiz-Valdepeñas Montiel, Víctor ; Gamella, Maria ; Pingarrón, José M ; García-García, Aina
2025-04-01·Data in Brief
Dataset for common wheat (Triticum aestivum L.) grain and flour characterization using classical and advanced analyses
Article
作者: Deborde, Catherine ; Chambrey, Baptiste ; Le Gall, Sophie ; Munch, Mélanie ; Kansou, Kamal ; Morel, Marie-Hélène ; Geoffroy, Sonia ; Buche, Patrice ; Linossier, Laurent ; Menut, Luc ; Rezette, Laura ; Weber, Magalie ; Saulnier, Luc ; Meleard, Benoit ; Dervaux, Stéphane
2025-04-01·International Journal of Biological Macromolecules
Improving gliadin functionality by Maillard glycosylation using hydrolyzed starch molecules of different molecular mass: Structure-function relationship study
Article
作者: Moldakhmetova, Raushan ; Zhao, Qi ; Li, Wenhao ; Niu, Li ; Sun, Tianrui ; Wang, Xinyu ; Li, Ruijie
5
项与 醇溶蛋白 相关的新闻(医药)2024-11-19
The growing comprehension of the pathogenesis of celiac disease has paved the way for the exploration of alternative treatment strategies. These novel approaches are aimed at addressing the condition at its root cause, targeting the underlying mechanisms that trigger the immune response in individuals with celiac disease. These innovative approaches hold the promise of transforming the landscape of celiac disease treatment in the near future.
LAS VEGAS, Nov. 19, 2024 /PRNewswire/ -- DelveInsight's
'
Celiac Disease Pipeline Insight 2024
' report provides comprehensive global coverage of pipeline celiac disease therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the celiac disease pipeline domain.
Key Takeaways from the Celiac Disease Pipeline Report
DelveInsight's celiac disease pipeline report depicts a robust space with
25+ active players working to develop
30+ pipeline therapies for celiac disease treatment.
Key celiac disease companies such as
Takeda, Sanofi, Entero Therapeutics, Pfizer, Topas Therapeutics, Anokion SA, Protagonist Therapeutics, Equillium, Theriva Biologics, Chugai Pharmaceutical, Novartis, Immunic, Mozart Therapeutics, Nemysis Ltd, Barinthus Biotherapeutics, Parvus Therapeutics, Kallyope, AMYRA Biotech AG, Forte Biosciences, LAPIX Therapeutics, Ahead Therapeutics, Allero Therapeutics, STEMCELL Technologies, IM Therapeutics, Provid Pharmaceuticals, Sigmoid Pharma, Janssen Biotech, Inc., Lumen Bioscience, IGY Life Sciences, Ahead Therapeutics, ANTOLRX, Imcyse, and others are evaluating new celiac disease drugs to improve the treatment landscape.
Promising Celiac Disease pipeline therapies such as
TAK-101, TAK-062, PRV-015, Latiglutenase, TAK-227, Ritlecitinib, TPM-502, KAN 101, PTG-100, EQ 102, SYN-020, DONQ52, CALY-002, IMU-856, MTX-101, E 40-02, VTP-1000, PVT-301, Research Program: Celiac disease, AMY02, FB 102, LPX-TIGI, AT 1718, ALL-001, SQZ TACs, Research Program: small molecule therapeutics, Research Program: DQ2/8, Research Program: SmPillformulations, Research Program: autoimmune disease therapeutics, IgY-112, AT 1715, Research programme: Celiac disease, and others are under different phases of Celiac Disease clinical trials.
In October 2024, Topas Therapeutics announced positive topline results from its Phase IIa trial of TPM502, in patients with celiac disease. The study data serves as the first clinical proof of concept for Topas' proprietary nanoparticle platform and its potential to induce targeted, antigen-specific tolerogenic effects.
In September 2024, Barinthus Biotherapeutics plc, a clinical-stage biopharmaceutical company developing novel immunotherapeutic candidates that guide T cells to control disease, announced the initiation of its first-in-human Phase I trial of VTP-1000 in adults with coeliac disease.
In May 2024, Entero Therapeutics, Inc. (formerly First Wave BioPharma, Inc.) unveiled its new corporate name and website. The rebranding follows the recent business combination with ImmunogenX and reflects the Company's focus on addressing unmet needs in GI health that include celiac disease, an indication for which there are no approved medicines today.
In March 2024, First Wave BioPharma announced the acquisition of ImmunogenX in an all-stock transaction with the combined company focused on advancing a GI pipeline comprised of multiple, late-stage clinical assets, including latiglutenase, a potentially first-in-class, near Phase III-ready, targeted, oral biotherapeutic for celiac disease.
In February 2024, with the approval of the SQZ transaction, STEMCELL acquired substantially all of SQZ's assets including its entire portfolio of over 400 patents and trademarks, other intellectual property such as copyrights and trade secrets, proprietary equipment, and its head license with the Massachusetts Institute of Technology.
In January 2024, Calypso Biotech BV announced that it had entered into an agreement to be acquired by Novartis AG ('Novartis'). Calypso's shareholders received an upfront payment of $250 million upon closing and are eligible to receive development milestones of up to $175 million based on the achievement of certain predetermined milestones. The acquisition gives Novartis full rights to CALY-002. Novartis intends to further explore CALY-002 across a wide variety of autoimmune indications with high unmet medical needs.
Request a sample and discover the recent advances in celiac disease treatment drugs @
Celiac Disease Pipeline Report
The celiac disease pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage celiac disease drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the celiac disease clinical trial landscape.
Celiac Disease Overview
Celiac disease is a chronic autoimmune disorder triggered by the ingestion of gluten, a protein found in wheat, barley, and rye. In individuals with celiac disease, the immune system mistakenly attacks the small intestine upon gluten consumption, leading to inflammation and damage to the intestinal lining, specifically the villi, which are essential for nutrient absorption. The exact cause of celiac disease is not fully understood, but it is known to involve a combination of genetic predisposition (usually associated with HLA-DQ2 or HLA-DQ8 genes) and environmental factors.
Common symptoms of celiac disease include digestive issues such as diarrhea, bloating, gas, constipation, and abdominal pain. However, some individuals experience non-digestive symptoms, like fatigue, anemia, joint pain, skin rashes, and even neurological issues such as headaches or depression. Because of this broad symptom range, celiac disease is often underdiagnosed or misdiagnosed as other conditions.
Diagnosis typically involves blood tests to detect specific antibodies (like tissue transglutaminase antibodies) and, if positive, is followed by an intestinal biopsy to confirm damage to the villi. Genetic testing may also be conducted in some cases to identify predispositions.
The primary treatment for celiac disease is a strict, lifelong gluten-free diet. By avoiding gluten, individuals with celiac disease can heal intestinal damage and prevent symptoms and complications. However, adherence to this diet requires vigilance, as even small amounts of gluten can trigger symptoms and damage.
Find out more about celiac disease treatment drugs @
Drugs for
Celiac Disease Treatment
A snapshot of the Celiac Disease Pipeline Drugs mentioned in the report:
Learn more about the emerging celiac disease pipeline therapies @
Celiac Disease Clinical Trials
Celiac Disease Therapeutics Assessment
The celiac disease pipeline report proffers an integral view of the celiac disease emerging novel therapies segmented by stage, product type, molecule type, mechanism of action, and route of administration.
Scope of the Celiac Disease Pipeline Report
Coverage: Global
Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
Therapeutics Assessment
By Route of Administration: Oral, Intravenous, Subcutaneous, Parenteral, Topical
Therapeutics Assessment
By Molecule Type: Recombinant fusion proteins, Small molecule, Monoclonal antibody, Peptide, Polymer, Gene therapy
Therapeutics Assessment
By Mechanism of Action: Gliadin inhibitors, Gluten modulators, Transglutaminase 2 inhibitors, Emt protein-tyrosine kinase inhibitors, Janus kinase 3 inhibitors, Alpha4beta7 integrin antagonists, Interleukin 15 inhibitors, Interleukin 21 inhibitors, Alkaline phosphatase replacements, HLA-DQ antigen inhibitors
Key Celiac Disease Companies: Takeda, Sanofi, Entero Therapeutics, Pfizer, Topas Therapeutics, Anokion SA, Protagonist Therapeutics, Equillium, Theriva Biologics, Chugai Pharmaceutical, Novartis, Immunic, Mozart Therapeutics, Nemysis Ltd, Barinthus Biotherapeutics, Parvus Therapeutics, Kallyope, AMYRA Biotech AG, Forte Biosciences, LAPIX Therapeutics, Ahead Therapeutics, Allero Therapeutics, STEMCELL Technologies, IM Therapeutics, Provid Pharmaceuticals, Sigmoid Pharma, Janssen Biotech, Inc., Lumen Bioscience, IGY Life Sciences, Ahead Therapeutics, ANTOLRX, Imcyse, and others.
Key Celiac Disease Pipeline Therapies: TAK-101, TAK-062, PRV-015, Latiglutenase, TAK-227, Ritlecitinib, TPM-502, KAN 101, PTG-100, EQ 102, SYN-020, DONQ52, CALY-002, IMU-856, MTX-101, E 40-02, VTP-1000, PVT-301, Research Program: Celiac disease, AMY02, FB 102, LPX-TIGI, AT 1718, ALL-001, SQZ TACs, Research Program: small molecule therapeutics, Research Program: DQ2/8, Research Program: SmPillformulations, Research Program: autoimmune disease therapeutics, IgY-112, AT 1715, Research programme: Celiac disease, and others.
Dive deep into rich insights for new drugs for celiac disease treatment, visit @
Celiac Disease Drugs
Table of Contents
For further information on the celiac disease pipeline therapeutics, reach out @
Celiac Disease Treatment Drugs
Related Reports
Celiac Disease Epidemiology
Celiac Disease Epidemiology Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted celiac disease epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Celiac Disease Market
Celiac Disease Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, market share of the individual therapies, and key celiac disease companies including
Entero Therapeutics, Amgen, Provention Bio, Takeda, among others.
Sjogren's Syndrome Market
Sjogren's Syndrome Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key Sjogren's syndrome companies including
Horizon Therapeutics (Amgen), Dompe Farmaceutici, Sylentis, MorphoSys, Resolve Therapeutics, OSE Immunotherapeutics, Servier, Novartis, Johnson & Johnson
, among others.
Sjogren's Syndrome Pipeline
Sjogren's Syndrome Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key Sjogren's syndrome companies, including
Novartis, Horizon Therapeutics, Bristol-Myers Squibb, Rise Therapeutics, Resolve Therapeutics, Dompe Farmaceutici, among others.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve
.
Contact Us
Shruti Thakur
[email protected]
+14699457679
Logo:
SOURCE DelveInsight Business Research, LLP
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
440k+
Newsrooms &
Influencers
9k+
Digital Media
Outlets
270k+
Journalists
Opted In
GET STARTED

临床结果临床2期并购临床1期免疫疗法
2022-07-29
·生物谷
时间匆匆易逝,转眼间7月份即将结束,在即将过去的7月里,Nature杂志又有哪些亮点研究值得学习呢?小编对相关文章进行了整理,与大家一起学习!
时间匆匆易逝,转眼间7月份即将结束,在即将过去的7月里,Nature杂志又有哪些亮点研究值得学习呢?小编对相关文章进行了整理,与大家一起学习!
【1】Nature:线粒体中的RNA修饰或能促进癌症的侵袭性扩散
doi:10.1038/s41586-022-04898-5
线粒体是细胞中的能量工厂,其含有自己的遗传物质和RNA分子,近日,一篇发表在国际杂志Nature上题为“Mitochondrial RNA modifications shape metabolic plasticity in metastasis”的研究报告中,来自德国癌症研究中心等机构的科学家们通过研究发现,线粒体RNA发生的特性修饰或许会通过支持线粒体蛋白质的合成从而增强癌细胞的侵袭性扩散;如今研究者已经确定,与高水平线粒体RNA修饰相关的特定的基因表达特征或与人类头颈癌的转移以及较差的预后相关,当研究人员阻断癌细胞中负责RNA修饰的酶类的功能后,发生转移的癌细胞的数量减少了,而且抑制线粒体中蛋白质合成的特定抗生素或许还能在实验室环境中抑制癌细胞的侵袭性扩散。
侵袭性肿瘤中的癌细胞能入侵周围组织并试图在其它器官中形成一种新的肿瘤,在这一旅程中,癌细胞就必须在不利的条件下存活,诸如氧气短缺或缺少营养物质等,为了克服这些压力因子,癌细胞就必须相应地调整其能量产生,目前研究人员并不清楚允许这种灵活性背后的分子机制,然而,研究人员推测,这种代谢可塑性或许就是癌细胞成功扩散的关键。机体呼吸链组分的产生受到了线粒体中特定细胞机器的严格调控,这对于癌细胞的转移性扩散具有非常重要的意义,文章中,研究者指出,tRNA或许就是该细胞机器的一部分,其主要负责在蛋白质组装的过程中提供单个氨基酸构件,研究人员识别出线粒体tRNAs上的分子修饰或许能作为支持在癌细胞转移期间蛋白质产生的控制机制。
癌细胞的入侵是一个非常消耗能量的过程,研究人员发现,线粒体tRNA中发现的特定化学修饰—m5C(5-甲基胞嘧啶,5-methylcytosine)或许是癌症转移发生所需要的,m5C修饰能促使线粒体中蛋白质的合成增加,这或许就会增强呼吸链组分的产生,因此,细胞就会增加其能量存储池从而为要求更高的细胞过程提供能量,比如癌细胞从肿瘤中的扩散。从另一方面来讲,缺失m5C的癌细胞或许能通过一种相对不太有效的机制(糖酵解)来获取能量,且其转移能力非常有限,研究者在小鼠机体中生长的人类肿瘤中证实了这一点,然而,原发性肿瘤的细胞活力或生长或许并不会收到m5C缺失的影响。
图片来源:https://pubmed-ncbi-nlm-nih-gov.libproxy1.nus.edu.sg/35768510/
【2】Nature:间歇性禁食竟可促进神经损伤愈合
doi:10.1038/s41586-022-04884-x
在一项新的研究中,来自英国帝国理工学院的研究人员发现间歇性禁食改变了小鼠的肠道细菌活动,增加了它们从神经损伤中恢复的能力。相关研究结果于2022年6月22日在线发表在Nature期刊上,论文标题为“The gut metabolite indole-3 propionate promotes nerve regeneration and repair”。
这些作者观察到间歇性禁食如何导致肠道细菌增加一种称为3-吲哚丙酸(3-Indolepropionic acid, IPA)的代谢物的产生。这种代谢物是称为轴突的神经纤维再生所需要的,而轴突是神经细胞末端的线状结构,向身体的其他细胞发出电化学信号。
这种新的机制是在小鼠身上发现的,并希望在未来的任何人体临床试验中也能得到证实。这些作者表示产生IPA的肠道细菌,即产芽孢梭菌(Clostridium sporogenesis),在人类和小鼠的肠道中自然存在,而且IPA也存在于人类的血液中。
论文通讯作者、帝国理工学院脑科学系Simone Di Giovanni教授说,“目前除了手术重建之外,没有任何治疗神经损伤的方法,而手术重建只对一小部分病例有效,这促使我们研究生活方式的改变是否可以有助于恢复。其他研究之前已将间歇性禁食与伤口修复和新神经元的生长联系在一起---但我们的研究是第一项明确解释禁食如何有助于治愈神经损伤的研究。”
【3】Nature:震惊!诺如病毒和轮状病毒等肠道病毒可通过唾液传播
doi:10.1038/s41586-022-04895-8
在一项新的研究中,来自美国国家卫生研究院(NIH)等研究机构的研究人员发现一类已知会导致严重腹泻疾病的肠道病毒可以在小鼠的唾液腺中生长,并通过其唾液传播。这一发现表明,这些常见的肠道病毒存在一种新的传播途径。这些肠道病毒每年困扰着全世界数十亿人,而且可能是致命的。相关研究结果于2022年6月29日在线发表在Nature期刊上,论文标题为“Enteric viruses replicate in salivary glands and infect through saliva”。
这些所谓的肠道病毒通过唾液传播表明,咳嗽、说话、打喷嚏、分享食物和餐具,甚至接吻都有可能传播这些病毒。这些新发现仍然需要在人类研究中得到证实。这些发现可能会带来更好的方法来预防、诊断和治疗由这些病毒引起的疾病,并有可能拯救生命。
一段时间以来,科学家们已经知道,诸如诺如病毒和轮状病毒之类的肠道病毒可以通过摄入被含有这些病毒的粪便污染的食物或液体而传播。肠道病毒被认为会绕过唾液腺,以肠道为目标,随后通过粪便排出。尽管一些科学家怀疑可能存在另一种传播途径,但直到现在这一理论在很大程度上仍未得到验证。
如今,这些作者将需要证实肠道病毒的唾液传播在人类中是可能的。他们说,如果他们发现确实如此,他们还可能发现这种传播途径甚至比传统途径更常见。他们说,这样的发现可能有助于解释为什么全世界每年肠道病毒感染的数量如此之多,却未能充分说明粪便污染是唯一的传播途径。
【4】Nature:重磅!科学家识别出一种能增强脂肪燃烧产热的特殊分子
doi:10.1038/s41586-022-05041-0
通常情况下,脂肪细胞能储存能量,然而,在棕色脂肪细胞中,能量会以热量的形式消散,因此棕色脂肪细胞就充当了生物加热器的角色,因此,在大多数哺乳动物机体中都存在这种机制,在人类机体中,其能让新生儿保持温暖,而在成年人机体中,棕色脂肪的激活或许与机体心脏代谢健康呈现正相关关系。
近日,一篇发表在国际杂志Nature上题为“Apoptotic brown adipocytes enhance energy expenditure via extracellular inosine”的研究报告中,来自波恩大学等机构的科学家们通过研究发现了增强机体中脂肪燃烧的特殊分子。研究者表示,我们确定了一种名为肌苷(inosine)的关键分子,其能帮助燃烧脂肪;众所周知,死亡的细胞会释放一种能影响其邻居细胞功能的信使分子混合物,于是研究人员想通过研究了解是否这种机制也存在于棕色脂肪中。随后研究人员对遭受严重压力的棕色脂肪细胞进行研究,这些细胞实际上正在发生死亡,结果发现,这些细胞会大量分泌嘌呤肌苷(purine inosine),有意思的是,完整的棕色脂肪细胞如何响应分子求救信号呢?其能被肌苷(或者是附近的死亡细胞)所激活,肌苷实际上扮演着“煽风点火”的角色,白色脂肪细胞会转化为棕色脂肪细胞,相比对照动物而言,被喂食高能量饮食并同时利用肌苷处理的小鼠仍然会变得很瘦,且能免受糖尿病的侵害。
肌苷转运蛋白似乎在这一背景下扮演着非常重要的角色,细胞膜中的特殊蛋白会将肌苷转运到细胞中,从而就会降低细胞外的浓度,因此,肌苷或许就不再会发挥其助燃的效应。研究者Pfeifer说道,有一种药物实际上是为了治疗凝血障碍所开发的,但其也会抑制肌苷转运蛋白。研究人员给予小鼠服用这种药物,结果发现其或许能燃烧更多的能量,人类机体中也存在这种肌苷转运蛋白,在大约2%-4%的人群中,由于遗传突变,其或许会变得不太活跃;研究人员对900名个体进行相关的遗传分析,结果发现,那些肌苷转运蛋白活性较低的受试者或许会平均明显更瘦弱一些。
图片来源:https://pubmed-ncbi-nlm-nih-gov.libproxy1.nus.edu.sg/35790189/
【5】Nature:利用DNA打字机读取细胞内的信息
doi:10.1038/s41586-022-04922-8
在一项新的研究中,华盛顿大学遗传学者Jay Shendure和他的团队利用他们开发出的一种新的细胞内记录系统来编码文本,对它进行测试。由于他们的发明依赖于一种几乎全新的记录媒介--DNA,他们希望使用能够唤起历史意义的信息。他们把这种系统命名为DNA打字机(DNA typewriter)。相关研究结果于2022年7月6日在线发表在Nature期刊上,论文标题为“A time-resolved, multi-symbol molecular recorder via sequential genome editing”。
虽然DNA打字机证明了它可以将短语像秘密信息一样塞进细胞,但它最强大的潜力在于其他方面。Shendure和他的同事们设想,科学家们将利用这项技术来记录细胞的内部运作。使用DNA来编码信息的想法是借助于这种分子的天然功能。就像计算机软件是用1和0写成的一样,DNA分子使用四个碱基的代码作为生物体内的指令。
DNA打字机并不是第一个这样的打字机,但是,它有多种属性的组合,使它在阐明细胞生物学方面特别有希望。也就是说,它可以捕获大量事件,同时按时间顺序记录它们。Shendure说,“我们完成了类似于写作的事情。我们可以创建数以千计的符号,我们称之为条形码,并且我们可以按顺序捕捉它们。”
【6】Nature:重大进展!发现调节肠道上皮中干细胞的新机制
doi:10.1038/s41586-022-04962-0
覆盖在小肠和大肠内部的一层特殊细胞吸收营养物和水,同时阻止任何有害物质循环。这层细胞被称为肠道上皮。它每四到七天利用肠道干细胞完全自我更新。但是,科学家们仍然不知道这些干细胞究竟是如何发挥作用的。在一项新的研究中,来自荷兰癌症研究所、奥地利科学技术研究所、芬兰赫尔辛基大学、瑞典卡罗琳学院和英国剑桥大学的研究人员研究了肠道上皮中的干细胞。他们发现了一个令人兴奋的新机制,可能改变人们对什么是干细胞的理解,相关研究结果于2022年7月13日在线发表在Nature期刊上。
肠道上皮只有一层细胞的厚度,并不断地更新。它遍布绒毛,这些绒毛看起来像覆盖在小肠和大肠内部的微小触角。在绒毛之间,有称为肠隐窝(intestinal crypt)的微小口袋。这个名字让人感到神秘,这很好地描述了那里到底发生了什么。论文共同第一作者Bernat Corominas-Murtra解释说,“在隐窝的底部,肠道上皮中的干细胞不断分裂。所产生的一些细胞作为干细胞留在隐窝中,其他细胞则被推向周围绒毛的顶端,在那里,它们最终分化为让肠道发挥功能的功能性细胞类型,并在几天后被丢弃。这种情况一直在人体内发生,如果这种机制崩溃,人体会遇到严重的医学问题。”
在研究小肠和大肠中的这些干细胞时,这些作者最初感到很困惑。Corominas-Murtra说,“我们通常对干细胞的看法是,干细胞是由细胞固有的生化特性决定的---类似于我们可以识别的生化标志物一样。我们发现在具有经典干细胞标志物的细胞中,许多细胞实际上从未作为干细胞发挥作用,而是被挤出隐窝并被丢弃,对肠道的长期更新没有任何贡献。我们还看到,虽然经典标志物预测小肠和大肠中的干细胞数量大致相同,但小肠中实际作为干细胞发挥作用的干细胞数量约为大肠中的两倍。”
【7】Nature:新研究有助解释为何人们不对食物产生免疫反应
doi:10.1038/s41586-022-04916-6
在一项新的研究中,来自美国明尼苏达大学医学院的研究人员研究了为什么大多数人在摄入食物后不产生免疫反应的问题。他们试图通过对小鼠进行实验来回答这个问题。相关研究结果于2022年7月5日在线发表在Nature期刊上。人类的免疫系统已经进化到产生各种反应,旨在使进入身体的外来成分变得无害。最常见的反应之一是触发有时被视为与过敏有关的症状:炎症。但是,正如这项新研究所指出的那样,为什么免疫系统不对人体每天摄入的食物产生反应呢?毕竟,它们都是外来物。
这些实验涉及到首先用无麸质饮食将几只实验室小鼠从幼鼠养大。接下来,它们被给予不同的食物,以使得这些作者能够观察到它们的免疫系统将如何作出反应。所测试的一些主要食物是那些含有一种或多种被称为麦醇溶蛋白(gliadin)的麸质蛋白类型的食物---先前的研究已表明这类食物往往在人类和小鼠中引起免疫反应。
这些作者将不同类型的食物喂给这些受试小鼠一周,然后测量它们产生的免疫反应。他们发现,在摄入含有麦醇溶蛋白的食物后,这些小鼠肠道中的T细胞数量略有增加,而且其中的一小部分T细胞产生了微弱的抗体反应---值得注意的是,它们中的许多是调节性T细胞(Treg细胞),它们往往具有免疫抑制作用,这可能部分上解释了对肠道中发现的食物普遍没有反应。他们还发现一些T细胞似乎与通常在免疫反应中发现的T细胞类型不同,尽管他们指出,它们可能是Treg细胞的前体细胞。在他们的实验中,他们发现在肠道中对食物的存在作出反应的T细胞中没有一种是可以触发炎症的T细胞类型。
在摄入食物后,麦醇溶蛋白抗体的产生依赖于Tfh细胞。
图片来源:Nature, 2022, doi:10.1038/s41586-022-04916-6。
【8】Nature:重磅!破解人类卵母细胞在卵巢中休眠50年仍具生殖能力之谜
doi:10.1038/s41586-022-04979-5
在一项新的研究中,来自西班牙巴塞罗那科学技术研究所和庞培法布拉大学等研究机构的研究人员发现未成熟的人类卵细胞跳过了一种被认为是产生能量必不可少的基本代谢反应。相关研究结果于2022年7月20日在线发表在Nature期刊上。
通过改变代谢活动,未成熟的人类卵细胞可以避免产生有害的活性氧分子,这些分子可以累积起来,损害DNA并导致细胞死亡。这些发现解释了人类卵细胞如何在卵巢中保持长达50年的休眠状态而不丧失其生殖能力。
论文第一作者、巴塞罗那科学技术研究所基因组调节中心博士后研究员Aida Rodriguez博士说,“人类在出生时就拥有了一生中所有的卵细胞供应。由于人类也是最长寿的陆生哺乳动物,卵细胞必须保持原始状态,同时避免几十年的磨损。我们表明,这个问题是通过跳过一种基本的代谢反应来解决的,这种代谢反应也是细胞损伤的主要来源。作为一种长期维护策略,它就像把电池置于待机模式。这代表了一种在动物细胞中从未见过的全新模式。”
在胎儿发育过程中,人类卵细胞(也称为卵子)首先在卵巢中形成,经历了不同的成熟阶段。在这个过程的早期阶段,被称为卵母细胞(oocyte)的未成熟卵细胞进入细胞停滞期,在卵巢中保持长达50年的休眠状态。像所有其他真核细胞一样,卵母细胞有线粒体---细胞的能量工厂---它们在这段休眠期利用线粒体来产生能量以满足其需求。
【9】Nature:首次构建出人类性腺发育的细胞图谱,鉴定出参与人类性别决定的细胞类型
doi:10.1038/s41586-022-04918-4
在一项新的研究中,来自英国韦尔科姆基金会桑格研究所等研究机构的研究人员绘制出首个人类两性性腺发育的大规模细胞图谱,作为绘制人体所有细胞类型的人类细胞图谱(Human Cell Atlas, HCA)计划的一部分。他们鉴定出新的细胞类型,包括那些表达“性别决定”基因的细胞类型,它们开始了决定一个人是否会成为表型上的男性或女性的过程。该细胞图谱将在改善生育治疗中的配子培养和理解生殖条件(比如性别发育差异)方面具有革命性的意义。相关研究结果于2022年7月6日在线发表在Nature期刊上。
性腺在人类发育中起着关键作用。在成熟为女性的卵巢或男性的睾丸之前,它们决定了生物性别,产生生殖所需的卵子和精子。发育的早期几周是非常动态的,这一过程中伴随着细胞类型迅速出现和消失。这使得研究导致性别决定和随后分化为睾丸或卵巢特异性细胞的事件具有挑战性。虽然我们对性腺发育的大部分知识来自于小鼠研究,但不确定这些知识有多少能真正转化为人类。
在这项新的研究中,这些作者着手绘制第一个大规模的人类两性性腺发育的细胞图谱,以表征细胞在生命早期成为睾丸或卵巢的路线。他们利用单细胞测序和空间转录组学分析了人类性腺组织的大约50万个细胞,涵盖了怀孕的第6至21周。他们还在小鼠中生成了一个类似的细胞图谱,他们用它来了解人类和小鼠生物学的相同或不同之处。迄今为止,这是在时间和空间方面最详细的发育中的性腺图谱。
人类和小鼠性腺和性腺外组织单细胞图谱。
图片来源:Nature, 2022, doi:10.1038/s41586-022-04918-4
【10】Nature:利用新方法揭示小鼠35种神经元亚型的特性
doi:10.1038/s41586-022-04915-7
在一项新的研究中,来自伦敦大学学院、牛津大学和哥伦比亚大学的研究人员开发出一种进行转录组学分析的新方法,并利用它揭示了小鼠中35种神经元亚型的特性。相关研究结果于2022年7月6日在线发表在Nature期刊上。
生物科学家的目标之一是了解大脑如何在其他动物和人类中发挥作用。考虑到大脑的复杂性,这个目标肯定是雄心勃勃的。但是,科学家们认为,这可以通过开展各种循序渐进的研究来完成,这些研究旨在解释特定大脑的各个部分,描述它们的作用。这项新研究的一部分涉及构建一个脑细胞类型和亚型的目录。细胞类型分类的一个方面涉及破译和记录全部表达的基因---这一过程被称为转录组学。由此可以确定每个单独的基因在大脑回路中发挥的作用。为此,科学家们使用了各种技术来进行转录组学(必须在活体组织上进行)分析,以了解更多关于大脑区域的情况,如小鼠视觉皮层。
在这项新的研究中,这些作者开发出一种新的方法来开展这样的研究工作--他们称之为coppaFISH,它能够从薄薄的脑组织切片中一次确定72个基因的表达。它涉及将体内双光子钙成像与传统的转录组学方法相结合。然后,由此产生的基因表达谱可用于将转录组映射到细胞身份上。(生物谷Bioon.com)
更多精彩阅读:
6月Nature杂志不得不看的重磅级亮点研究!
5月Nature杂志不得不看的重磅级亮点研究!
基因疗法抗体细胞疗法
2022-07-22
·生物谷
在一项新的研究中,来自美国明尼苏达大学医学院的研究人员研究了为什么大多数人在摄入食物后不产生免疫反应的问题。他们试图通过对小鼠进行实验来回答这个问题。相关研究结果于2022年7月5日在线发表在Nature期刊上,论文标题为“Immune tolerance of food is mediated by layers of CD4+ T cell dysfunction”。人类的免疫系统已经进化到产生各种反应,旨在使进入身体的外来成分变得无害。最常见的反应之一是触发有时被视为与过敏有关的症状:炎症。但是,正如这项新研究所指出的那样,为什么免疫系统不对人体每天摄入的食物产生反应呢?毕竟,它们都是外来物。这些实验涉及到首先用无麸质饮食将几只实验室小鼠从幼鼠养大。接下来,它们被给予不同的食物,以使得这些作者能够观察到它们的免疫系统将如何作出反应。所测试的一些主要食物是那些含有一种或多种被称为麦醇溶蛋白(gliadin)的麸质蛋白类型的食物---先前的研究已表明这类食物往往在人类和小鼠中引起免疫反应。这些作者将不同类型的食物喂给这些受试小鼠一周,然后测量它们产生的免疫反应。他们发现,在摄入含有麦醇溶蛋白的食物后,这些小鼠肠道中的T细胞数量略有增加,而且其中的一小部分T细胞产生了微弱的抗体反应---值得注意的是,它们中的许多是调节性T细胞(Treg细胞),它们往往具有免疫抑制作用,这可能部分上解释了对肠道中发现的食物普遍没有反应。他们还发现一些T细胞似乎与通常在免疫反应中发现的T细胞类型不同,尽管他们指出,它们可能是Treg细胞的前体细胞。在他们的实验中,他们发现在肠道中对食物的存在作出反应的T细胞中没有一种是可以触发炎症的T细胞类型。在摄入食物后,麦醇溶蛋白抗体的产生依赖于Tfh细胞。图片来自Nature, 2022, doi:10.1038/s41586-022-04916-6。这些作者认为他们的实验表明,T细胞不攻击食物的原因是初始T细胞(naïve T cell)接触食物抗原导致不能触发炎症功能的T细胞亚群的产生,但是这些T细胞亚群仍然能够产生抑制炎症的T细胞。参考资料:Sung-Wook Hong et al. Immune tolerance of food is mediated by layers of CD4+ T cell dysfunction. Nature, 2022, doi:10.1038/s41586-022-04916-6.
分析
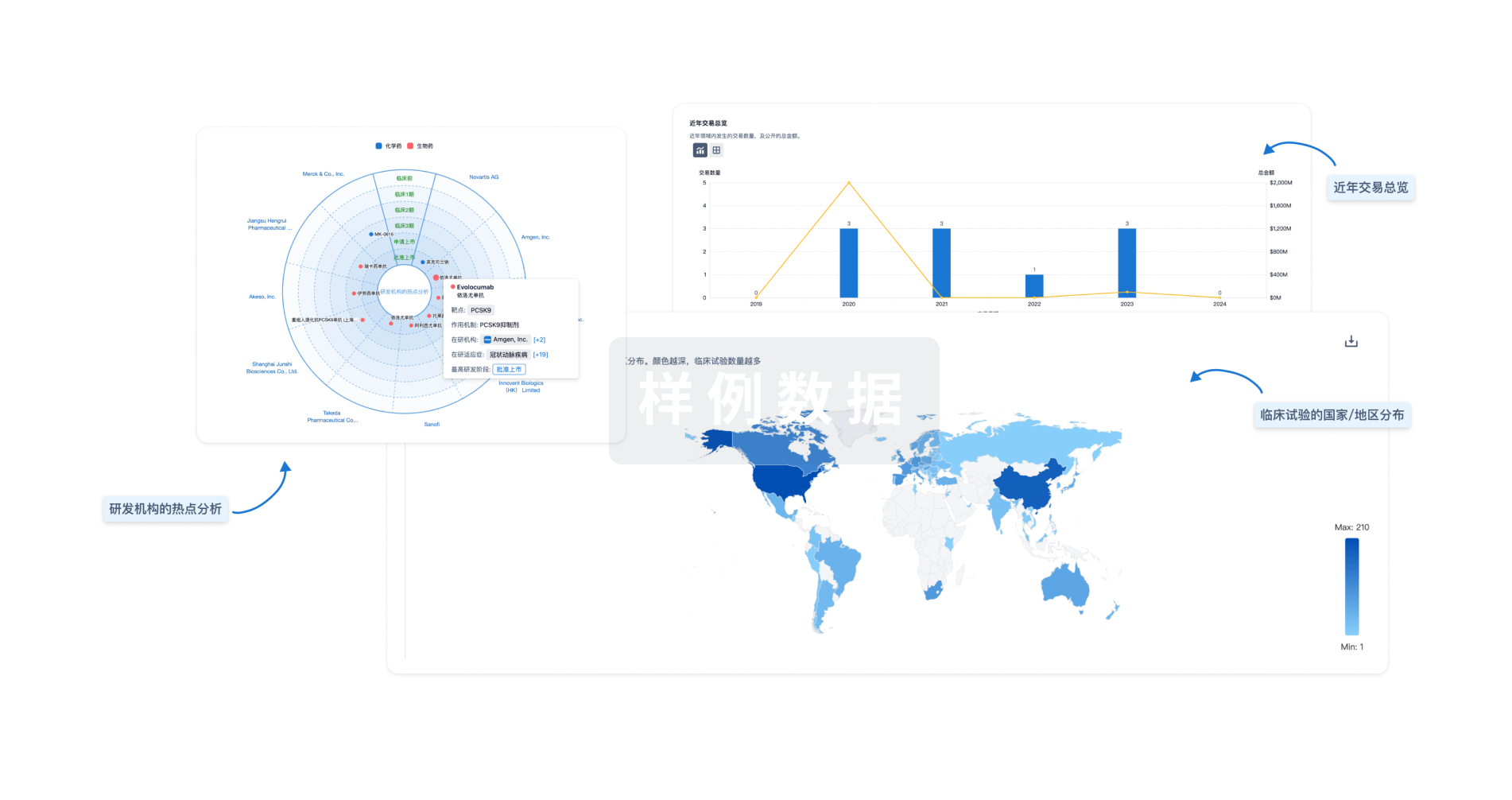
对领域进行一次全面的分析。
登录
或
生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用


