靶点- |
|
|
|
|
|
最高研发阶段批准上市 |
|
首次获批日期1963-09-25 |
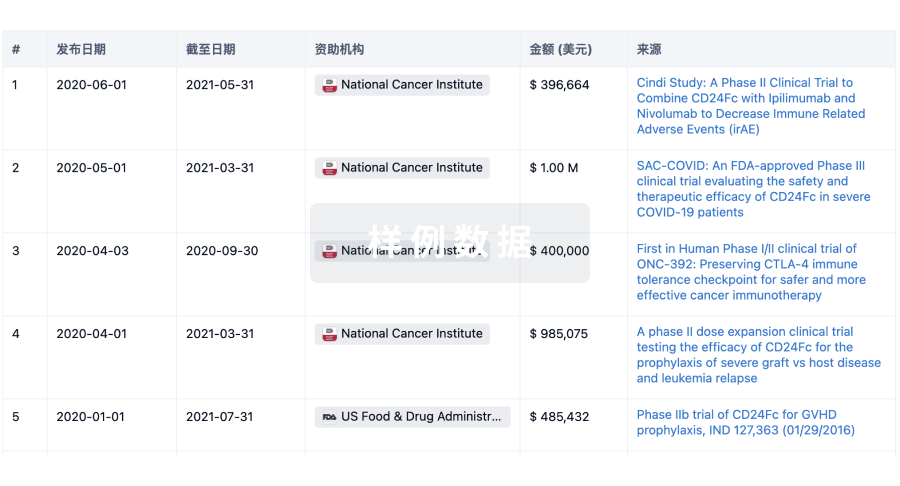
A Multi-Centre, Randomised, Open-Label Active-Controlled Study Comparing Safety and Efficacy of Synergo Radiofrequency (RF)-Induced Hyperthermia-Chemotherapy With Mitomycin C (RITE) Versus Bacillus Calmette-Guérin (BCG) as First-Line Treatment of Non-Muscle Invasive Papillary Bladder Cancer (NMIBC)
A multi-institutional, prospective, randomised, open-label, superiority, comparative, active-controlled, phase 3 study. The study will compare Synergo RF-induced hyperthermia-chemotherapy (SHTC) plus mitomycin C (MMC) to standard treatment of bacillus Calmette-Guérin (BCG) therapy as first-line adjuvant treatment for intermediate and high-risk NMIBC, and will evaluate recurrence and progression rate over two years of follow-up.
A Multicenter, Single-Arm Study Evaluating the Efficacy of Synergo® Radiofrequency-Induced Thermochemotherapy Effect With Mitomycin C in Non-Muscle Invasive Bladder Cancer Patients With BCG-refractory CIS
This multicenter, prospective, single-arm, phase 3 study will assess the proportion of disease-free patients, starting from administration of the first study treatment to at least 12-months after the first treatment, and up to 2 years (the latter only in patients choosing to participate in longer-term disease-free-survival data collection).
A Randomized Controlled Study Comparing Adjuvant Hyperthermia Treatment in Conjunction With Mitomycin C Versus BCG Immunotherapy (BCG) Adjuvant Treatment in Patients With Superficial Transitional Cell Carcinoma of the Bladder (STCCB)
The study is designed to compare the efficacy and safety of 2 treatment types for the prevention of tumor recurrence of superficial bladder cancer:
A combination of bladder wall heating and local chemotherapy (Synergo)
Bacillus Calmette-Guérin (BCG)
100 项与 Medical Enterprises Europe BV 相关的临床结果
0 项与 Medical Enterprises Europe BV 相关的专利(医药)
100 项与 Medical Enterprises Europe BV 相关的药物交易
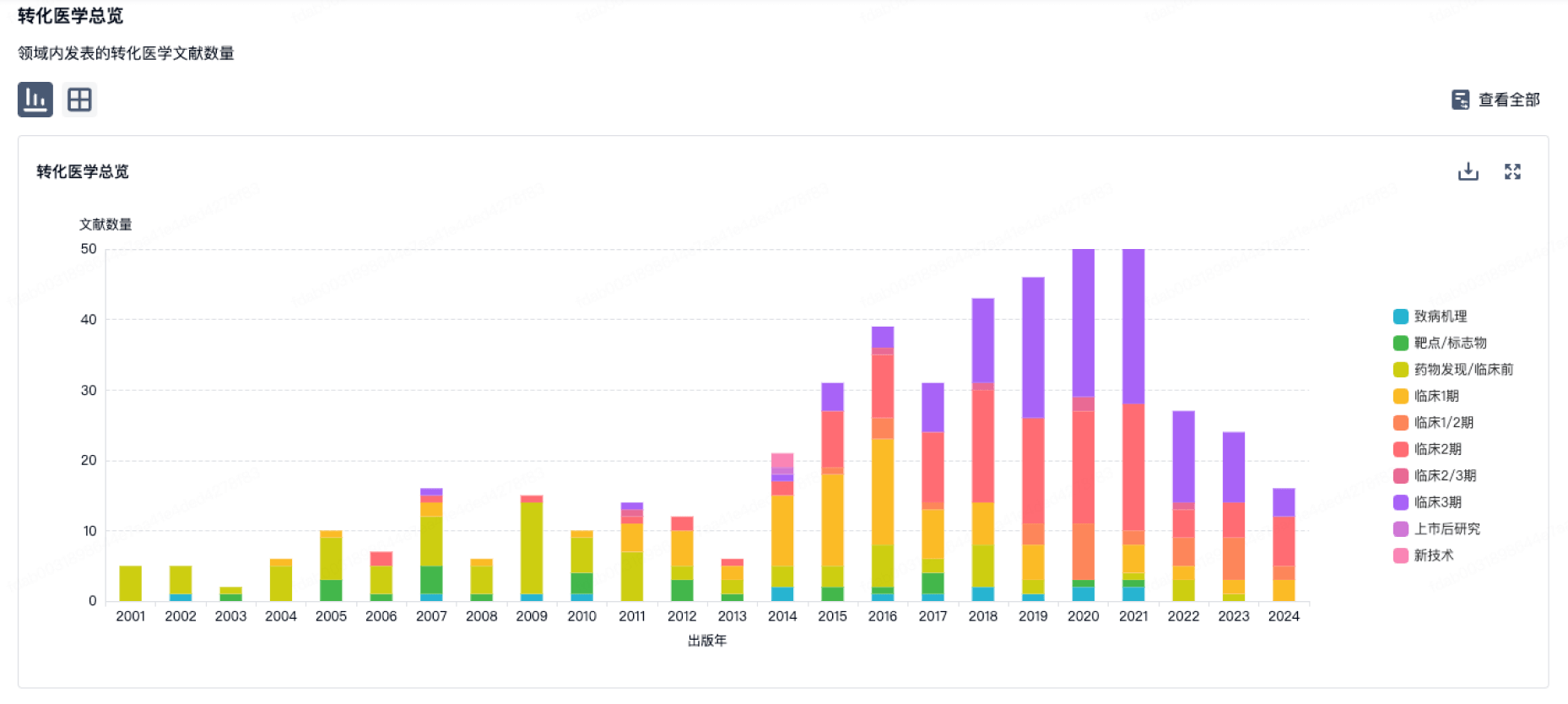
100 项与 Medical Enterprises Europe BV 相关的转化医学