预约演示
更新于:2025-08-29

United BioPharma, Inc.
更新于:2025-08-29
概览
标签
免疫系统疾病
肿瘤
皮肤和肌肉骨骼疾病
单克隆抗体
疾病领域得分
一眼洞穿机构专注的疾病领域
暂无数据
技术平台
公司药物应用最多的技术
暂无数据
靶点
公司最常开发的靶点
暂无数据
| 排名前五的药物类型 | 数量 |
|---|---|
| 单克隆抗体 | 5 |
关联
5
项与 联合生物制药股份有限公司 相关的药物靶点 |
作用机制 CD4抑制剂 [+1] |
在研机构 |
原研机构 |
在研适应症 |
非在研适应症- |
最高研发阶段临床3期 |
首次获批国家/地区- |
首次获批日期- |
靶点 |
作用机制 HER2拮抗剂 |
在研机构 |
原研机构 |
非在研适应症- |
最高研发阶段临床1期 |
首次获批国家/地区- |
首次获批日期- |
靶点 |
作用机制 HER2拮抗剂 |
在研机构 |
原研机构 |
在研适应症 |
非在研适应症- |
最高研发阶段临床前 |
首次获批国家/地区- |
首次获批日期- |
16
项与 联合生物制药股份有限公司 相关的临床试验NCT03149211
A Phase III, Randomized, Open-label, Controlled Trial to Investigate the Efficacy and Safety of UB-421 Monotherapy as Substitution for Stable Antiretroviral Therapy in HIV-1 Infected Adults
The purpose of this phase III study is to evaluate the efficacy, safety and tolerability of UB-421 monotherapy in suppressing viral rebound in HIV-1 infected adults undergoing antiretroviral treatment interruption.
开始日期2025-04-01 |
申办/合作机构 |
NCT04620291
A Phase I, Open-Label, Multi-Dose Study for Evaluation of the Safety, Pharmacokinetics, and Antiviral Activity of UB-421 Subcutaneous Formulation in HIV Infected Adults
UB-421 subcutaneous formulation (UB-421 SC) is developed to provide HIV infected patients a more convenient drug delivery method. UB-421 SC injection, with significantly less injection time than IV infusions and with opportunity of self-administration or administered in general medical setting (in addition to HIV-specific clinic), can provide patient a more convenient option.
This UB-421 SC phase I study will be conducted to investigate short-term safety, pharmacokinetics and anti-viral activity of UB-421 SC at three dose levels in ART-treated aviremic subjects and treatment naive HIV-infected subjects. The current UB-421 SC formulation (125 mg/ml) is at least 10-fold more concentrated than UB-421 IV (10 mg/ml). The highly concentrated formulation makes weekly UB-421 subcutaneous injections feasible. This study will form the basis of UB-421 SC in combination with antiretroviral agents (ARV) for treating HIV infected viremic patients in the future clinical trials.
This UB-421 SC phase I study will be conducted to investigate short-term safety, pharmacokinetics and anti-viral activity of UB-421 SC at three dose levels in ART-treated aviremic subjects and treatment naive HIV-infected subjects. The current UB-421 SC formulation (125 mg/ml) is at least 10-fold more concentrated than UB-421 IV (10 mg/ml). The highly concentrated formulation makes weekly UB-421 subcutaneous injections feasible. This study will form the basis of UB-421 SC in combination with antiretroviral agents (ARV) for treating HIV infected viremic patients in the future clinical trials.
开始日期2023-12-31 |
申办/合作机构 |
NCT04175704
A Phase I, Randomized, Single-Blind, Placebo-Controlled, Single Ascending Dose Study to Assess the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of UB-221 as an Add-on Therapy in Patients With Chronic Spontaneous Urticaria
The study is to evaluate the profiles of safety, tolerability, pharmacokinetics, and pharmacodynamics of UB-221. In this study, safety profile of UB-221 and maximum tolerated dose (MTD) is to be investigated using sentinel dosing strategy. The starting dose of 0.2 mg/kg is selected based on the evaluation and comparison of various approaches including NOAEL, MABEL (minimum anticipated biological effect level), and experiences from other anti-IgE mAb drugs in development.
开始日期2023-12-30 |
申办/合作机构 |
100 项与 联合生物制药股份有限公司 相关的临床结果
登录后查看更多信息
0 项与 联合生物制药股份有限公司 相关的专利(医药)
登录后查看更多信息
1
项与 联合生物制药股份有限公司 相关的文献(医药)2022-08-01·The Journal of clinical investigation
IgE-neutralizing UB-221 mAb, distinct from omalizumab and ligelizumab, exhibits CD23-mediated IgE downregulation and relieves urticaria symptoms
Article
作者: Lai, Annie ; Huang, Hong-Xuan ; Yang, Jasper ; Lynn, Shugene ; Shiung, Yu-Yu ; Lee, Chih-Hung ; Chen, Techeng ; Li, Chao-Hung ; Wang, Chang Yi ; Yang, Fu-Hung ; Liao, Mei-June ; Hsu, Cindy ; Lin, Chen-Han ; Tseng, William ; Tsai, Pei-Hua ; Cheng, Yi-Ning ; Liu, Yaw-Jen ; Kuo, Be-Sheng ; Su, Hsiao-Wen ; Chen, Jiun-Bo ; Wu, Ming-Syue ; Chang, Heng-Kwei ; Lung, Meng-Chung ; Li, Ywan-Feng ; Chu, Chia-Yu ; Lin, Chia-Yin ; Kuo, Je-Hung ; Ho, Yueh-Feng ; Wang, Yi-Jen
Over the last 2 decades, omalizumab is the only anti-IgE antibody that has been approved for asthma and chronic spontaneous urticaria (CSU). Ligelizumab, a higher-affinity anti-IgE mAb and the only rival viable candidate in late-stage clinical trials, showed anti-CSU efficacy superior to that of omalizumab in phase IIb but not in phase III. This report features the antigenic-functional characteristics of UB-221, an anti-IgE mAb of a newer class that is distinct from omalizumab and ligelizumab. UB-221, in free form, bound abundantly to CD23-occupied IgE and, in oligomeric mAb-IgE complex forms, freely engaged CD23, while ligelizumab reacted limitedly and omalizumab stayed inert toward CD23; these observations are consistent with UB-221 outperforming ligelizumab and omalizumab in CD23-mediated downregulation of IgE production. UB-221 bound IgE with a strong affinity to prevent FcԑRI-mediated basophil activation and degranulation, exhibiting superior IgE-neutralizing activity to that of omalizumab. UB-221 and ligelizumab bound cellular IgE and effectively neutralized IgE in sera of patients with atopic dermatitis with equal strength, while omalizumab lagged behind. A single UB-221 dose administered to cynomolgus macaques and human IgE (ε, κ)-knockin mice could induce rapid, pronounced serum-IgE reduction. A single UB-221 dose administered to patients with CSU in a first-in-human trial exhibited durable disease symptom relief in parallel with a rapid reduction in serum free-IgE level.
25
项与 联合生物制药股份有限公司 相关的新闻(医药)2025-03-21
February 2025 witnessed a wave of mergers and acquisitions in the global biopharmaceutical sector. From Pacira BioSciences acquiring GQ Bio Therapeutics GmbH for $32 million to accelerate the development of gene therapy, to Novartis making a $925 million investment to acquire Anthos Therapeutics and its potentially first-in-class drug abelacimab, the industry saw significant strategic moves. Meanwhile, Shen Lian Biomedical’s entry into the human innovative drug sector and Bosai Gene’s acquisition of certain assets of Skyline Therapeutics Limited further underscore the rising presence of Asian biotech companies on the global stage.
1.Pacira BioSciences Acquires GQ Bio to Advance Innovative Gene Therapies for Chronic Musculoskeletal Diseases
On February 27, 2025, Pacira BioSciences announced the acquisition of the remaining 81% equity of GQ Bio Therapeutics GmbH for a net purchase price of approximately $32 million. This acquisition not only strengthens Pacira’s leadership in non-opioid pain management but also aligns with its "5x30" strategy, aimed at becoming an innovative biopharmaceutical organization. Through this transaction, Pacira has integrated GQ Bio’s High-Capacity Adenovirus (HCAd) gene therapy vector platform, which is particularly well-suited for developing disease-modifying therapies for common musculoskeletal disorders.
The HCAd platform addresses key challenges in gene therapy, such as efficiently delivering large or multi-gene constructs into cells. Unlike many gene therapies that rely on Adeno-Associated Virus (AAV) vectors, the HCAd vector enables more effective gene delivery with significantly lower doses. Additionally, the HCAd platform can carry up to 30,000 base pairs of DNA, supporting multigenic or large-gene therapies. This allows for localized administration and repeat dosing at appropriate therapeutic intervals, significantly enhancing the feasibility and commercial appeal of gene therapy.
Pacira's lead candidate, PCRX-201 (enekinragene inzadenovec), originally developed by GQ Bio, leverages this innovative HCAd technology and is currently in clinical development for knee osteoarthritis. Administered via local injection into the knee joint, PCRX-201 promotes the cellular production of interleukin-1 receptor antagonist (IL-1Ra), thereby blocking the IL-1 pathway activation and reducing chronic inflammation. A key feature of PCRX-201 is its inflammation-responsive promoter, which ensures IL-1Ra is only produced when needed, mimicking the body's natural anti-inflammatory response. Preliminary clinical data suggest that PCRX-201 provides sustained relief for up to two years, improving pain, stiffness, and joint function while maintaining a favorable safety profile.
This acquisition brings both short-term and long-term financial benefits for Pacira, eliminating up to $64 million in future milestone payment obligations. Pacira intends to maintain GQ Bio’s operations, invest in its HCAd gene therapy vector platform, and develop innovative products based on this technology. Leveraging Pacira’s clinical, regulatory, and commercialization expertise, the company aims to maximize the potential of GQ Bio’s platform. Additionally, the two companies have identified multiple well-validated cytokines as potential foundations for additional localized gene therapies, further expanding the HCAd platform’s applications to address unmet medical needs.
Pacira remains committed to revolutionizing non-opioid pain management to enhance patient quality of life. The acquisition of GQ Bio and its HCAd platform not only solidifies Pacira’s leadership in musculoskeletal pain and related disorders but also offers new hope to millions of patients suffering from chronic pain—a condition affecting nearly one-quarter of the U.S. population. By tackling the underlying molecular causes of chronic pain, Pacira demonstrates its dedication and capability in addressing this national public health challenge.
2.Bosai Gene Acquires Certain Assets of Skyline Therapeutics Limited
Bosai Gene plans to acquire certain assets of Skyline Therapeutics Limited (Jiutian Cayman) using its own funds. This transaction aims to enhance the company’s sustainable development capabilities in the biotechnology sector. The acquisition scope includes assets of Jiutian Cayman and its subsidiaries, excluding its wholly owned subsidiary, Skyline Therapeutics (US) Inc., and any assets or rights it has obtained. Through this acquisition, Bosai Gene expects to integrate resources, drive technological innovation, and establish a stronger position in the international market.
The total transaction consideration consists of a base merger consideration of $15 million and an additional merger consideration of up to $43 million. The additional merger consideration is composed of development and sales milestone payments, sublicensing royalties, and sales royalties calculated as a single-digit percentage of annual net sales. All payments will be made in U.S. dollars, introducing a degree of foreign exchange risk due to fluctuations in the Renminbi to U.S. dollar exchange rate.
Jiutian Cayman specializes in the biotechnology field, possessing extensive R&D experience and multiple potentially high-value drug pipelines. Although the announcement does not specify particular drug names or mechanisms of action, considering the characteristics of the biotech industry, these drugs are likely to involve innovative therapies, such as gene therapy, cell therapy, or other advanced biologics. These therapies typically target specific molecular pathways to achieve therapeutic effects, offering breakthrough potential for certain refractory diseases. Through this acquisition, Bosai Gene stands to gain access to a range of competitive R&D projects and technology platforms, further enriching its product pipeline.
Upon completion of the acquisition, Jiutian Cayman will become a wholly owned subsidiary of Bosai Gene and will be consolidated into the company’s financial statements. However, differences in corporate culture and management practices between the two entities present integration challenges. To maximize synergies, Bosai Gene plans to implement a series of measures, including but not limited to internal controls, financial management, human resources management, and technical R&D support, ensuring the acquired company maintains a stable development trajectory. Furthermore, to retain key personnel, Bosai Gene has engaged in extensive discussions with critical employees of the target company and has established corresponding incentive mechanisms.
The transaction is subject to approval or filing with relevant government authorities and must be ratified by Jiutian Cayman’s shareholder meeting, requiring at least two-thirds of voting rights in favor for it to become effective. As a result, there remains uncertainty regarding the successful completion of the transaction. Additionally, risks associated with exchange rate fluctuations, goodwill impairment, key personnel departures, and other potential uncertainties must be considered. In response, Bosai Gene has formulated corresponding risk mitigation strategies and may adjust transaction terms if necessary to safeguard the company’s interests. As of the announcement date, relevant preparatory work is proceeding in an orderly manner.
3.Cosette Pharmaceuticals Acquires Mayne Pharma, Strengthening Leadership in Women’s Health and Dermatology
Cosette Pharmaceuticals, Inc. has announced that it has entered into a definitive agreement to acquire all issued shares of Mayne Pharma Group Limited at a price of AUD 7.40 per share, with a total transaction value of approximately $430 million. This acquisition has been approved by the boards of directors of both companies, and Mayne Pharma’s board has unanimously recommended that its shareholders vote in favor of the transaction. The deal is expected to close in Q2 2025. Through this acquisition, Cosette Pharmaceuticals aims to strengthen its business in women’s health and dermatology, while expanding its commercial, manufacturing, and geographical footprint.
The acquisition will establish Cosette Pharmaceuticals as a leading U.S.-based pharmaceutical company specializing in women’s health and dermatology, while also expanding its international market presence. The combined company will leverage the strengths of both firms in these specialized areas to drive innovation and enhance the accessibility of women’s health therapies. Notably, Cosette will acquire 12 patent-protected products, including well-known brands such as VYLEESI®, INTRAROSA®, NEXTSTELLIS®, and ANNOVERA®. For example, VYLEESI® is used to treat hypoactive sexual desire disorder (HSDD), while INTRAROSA® is prescribed for postmenopausal vaginal atrophy symptoms.
The combined entity will have a workforce of over 830 employees, including a highly effective and successful sales and marketing team, particularly in the specialized fields of women’s health and dermatology. Additionally, the company will own two state-of-the-art, FDA-approved manufacturing facilities—one located in Lincolnton, North Carolina, and the other in Salisbury, South Australia. These facilities will serve global patients, ensuring a consistent supply of high-quality pharmaceutical products. The acquisition will also enhance Cosette’s already robust product portfolio, reinforcing its market-leading commercial and operational capabilities.
Under the terms of the agreement, the acquisition will be executed via a scheme of arrangement, involving the 100% acquisition of all issued shares of Mayne Pharma. The transaction is expected to close in Q2 2025, subject to customary closing conditions, including regulatory and shareholder approvals. Upon completion, the combined company will be privately held. Both companies’ boards of directors have approved the transaction and have recommended that Mayne Pharma’s shareholders vote in favor of the deal, provided no superior offer emerges. Further details on the transaction can be found in Mayne Pharma’s announcement to the ASX.
4. FibroGen Announces Sale of Its China Subsidiary to AstraZeneca
On February 20, 2025, FibroGen, Inc. announced that it has agreed to sell its China subsidiary to AstraZeneca for approximately $160 million. The purchase price of this transaction includes an enterprise value of $85 million, along with FibroGen’s net cash holdings in China, currently estimated at approximately $75 million. This transaction not only strengthens FibroGen’s financial position but also extends its cash runway through 2027, enabling the company to continue advancing the clinical development of FG-3246 (an antibody-drug conjugate targeting CD46) and FG-3180 (a companion PET imaging agent) in metastatic castration-resistant prostate cancer (mCRPC).
Under the terms of the agreement, upon completion of the transaction, AstraZeneca will acquire all rights to roxadustat in China. Roxadustat is a leading brand in the treatment of anemia associated with chronic kidney disease (CKD), holding a significant market share, and is currently awaiting regulatory decisions for chemotherapy-induced anemia. Following the completion of this transaction, FibroGen will retain the rights to roxadustat in the U.S. and all markets not licensed to Astellas. The company plans to meet with the FDA in the second quarter of 2025 to discuss potential next steps for developing roxadustat for the treatment of anemia in patients with low-risk myelodysplastic syndromes (LR-MDS) in the U.S.
The transaction is expected to close in mid-2025, subject to customary closing conditions, including regulatory review in China. Upon completion, FibroGen will use a portion of the proceeds to repay a term loan provided by Morgan Stanley Tactical Value Investing Fund, further streamlining the company’s capital structure. Additionally, FibroGen reported unaudited total cash, cash equivalents, and accounts receivable of $121.1 million as of December 31, 2024. This indicates that, with this transaction, the company’s cash reserves will be sufficient to support its operations through 2027.
FibroGen will continue to advance its key assets, FG-3246 (also known as FOR46), a first-in-class antibody-drug conjugate targeting CD46, and its companion PET imaging agent, FG-3180. A Phase II clinical trial evaluating FG-3246 as a monotherapy for mCRPC patients is expected to commence in the second quarter of 2025. These research and development efforts are crucial for expanding the company’s oncology pipeline and demonstrating its commitment to developing innovative therapies.
5. Gate Neurosciences Strengthens Synapse-Targeted Therapeutics Through Acquisition of Boost Neuroscience
Gate Neurosciences announced that it has entered into a definitive agreement to acquire Boost Neuroscience and its leading proprietary platform, which focuses on human synaptic network analysis and the discovery and development of synapse-targeting therapeutics. This acquisition further strengthens Gate Neurosciences’ leadership in synapse science and drug development, providing cutting-edge insights into human synaptic network pharmacology. Boost Neuroscience was spun out from the laboratory of Dr. Thomas Südhof at Stanford University and was backed by Catalio Capital Management. Dr. Südhof was awarded the 2013 Nobel Prize in Physiology or Medicine for his research on synaptic transmission and biology.
Boost Neuroscience has developed a proprietary platform utilizing human induced pluripotent stem cells (iPSCs) to model neuronal network circuits, offering unprecedented insights into how molecules influence neuronal network formation, synaptogenesis, synaptic function, and neuroplasticity. The company aims to discover and develop novel therapies that enhance synaptic health and function to address a range of central nervous system (CNS) disorders. Based in Menlo Park, California, Boost Neuroscience’s laboratory and office space are equipped with state-of-the-art automated microscopy and robotics technology, complementing Gate Neurosciences’ existing translational research facility in Evanston, Illinois.
Through this acquisition, Gate Neurosciences will enhance its translational research capabilities in synaptic function pharmacology, further bolstering confidence in its internal clinical programs. Additionally, Boost Neuroscience’s early-stage pipeline will expand Gate Neurosciences’ portfolio by introducing new target programs. Notably, Boost’s proprietary platform is capable of identifying and validating targets and molecular scaffolds that enhance human synaptic function, offering potential treatments for a wide range of neuropsychiatric and neurodegenerative diseases driven by synaptic dysfunction.
Synaptic dysfunction and loss are well-established precursors to neuropsychiatric and neurodegenerative diseases. The addition of Boost Neuroscience will accelerate Gate Neurosciences’ efforts in translating synapse-targeting drug projects into successful therapies. Dr. Jacob Vogelstein, Managing Partner at Catalio Capital Management, remarked, “The Gate Neurosciences team has made significant progress in advancing translational pharmacology for synapse-targeted therapeutics.” He further emphasized that this integration will accelerate the development of a leading pipeline to treat major neurological and neuropsychiatric disorders driven by synaptic dysfunction.
6.Novartis Acquires Anthos Therapeutics for $925 Million to Strengthen Its Late-Stage Cardiovascular Pipeline
On February 11, 2025, Novartis announced an agreement to acquire Anthos Therapeutics, a Boston-based clinical-stage biopharmaceutical company developing abelacimab, a potentially first-in-class monoclonal antibody targeting the FXI inhibition pathway. This transaction aligns with Novartis’ growth strategy and therapeutic focus, leveraging the company’s expertise and leadership in the cardiovascular space. Under the terms of the agreement, Novartis will pay $925 million upfront upon transaction closing, with potential additional payments of up to $2.15 billion contingent upon achieving specific regulatory and sales milestones. The transaction is expected to close in the first half of 2025, subject to customary closing conditions.
Abelacimab is a novel, highly selective, fully human monoclonal antibody designed to provide effective anticoagulation with hemostasis preservation via Factor XI inhibition. Phase 2 data have demonstrated a significant reduction in bleeding events in atrial fibrillation (AF) patients treated with abelacimab compared to standard-of-care direct oral anticoagulants (DOACs). Currently, three Phase 3 clinical trials are underway, targeting patients at risk of arterial and venous thrombosis, including AF patients (LILAC-TIMI 763) and cancer-associated thrombosis (ASTER4 and MAGNOLIA5). Additionally, in 2022, abelacimab received Fast Track designation from the FDA for the treatment of cancer-associated thrombosis and the prevention of stroke and systemic embolism in AF patients.
Through this acquisition, Anthos Therapeutics will join Novartis, further strengthening its cardiovascular focus while complementing the company’s life-changing therapies, comprehensive clinical programs, and strategic collaborations. Dr. Shreeram Aradhye, President of Development and Chief Medical Officer at Novartis, stated, “We are thrilled to join forces to advance abelacimab, a potentially first-in-class therapy offering a safer approach for atrial fibrillation and cancer-associated thrombosis.” This acquisition not only enhances Novartis’ cardiovascular pipeline but also underscores its commitment to addressing the needs of cardiovascular patients worldwide.
As a potential first-in-class therapy, abelacimab aims to offer a more effective and safer approach to preventing thrombosis and stroke compared to the current standard of care. The drug works by selectively targeting Factor XI, a key protein in the blood coagulation cascade. By inhibiting Factor XI, abelacimab reduces unnecessary clot formation while preserving normal hemostatic function. This unique mechanism makes abelacimab highly promising for the prevention of stroke and systemic embolism in AF patients, as well as the treatment of cancer-associated thrombosis.
Under the terms of the agreement, in addition to the $925 million upfront payment, Novartis may pay up to $2.15 billion in milestone-based payments based on regulatory and commercial achievements. This acquisition reflects Novartis’ commitment to investing in innovative therapies and its long-term dedication to advancing cardiovascular disease treatments. With abelacimab now integrated into Novartis’ Cardiovascular, Renal, and Metabolism (CRM) development pipeline, the company looks forward to further clinical advancements that could unlock its full therapeutic potential.
7.Alumis and ACELYRIN Merge to Advance Transformative Therapies for Immune-Mediated Diseases
On February 6, 2025, Alumis Inc. and ACELYRIN, INC. announced a definitive merger agreement to combine in an all-stock transaction, forming a late-stage clinical biopharmaceutical company dedicated to the innovation, development, and commercialization of transformative therapies for immune-mediated diseases. The merged entity will operate under the Alumis name and be led by the current Alumis executive team. Under the agreement, shareholders of Alumis and ACELYRIN will own approximately 55% and 45% of the combined company, respectively (on a fully diluted basis). This merger not only provides a value-driven opportunity for shareholders but also marks a significant step forward in advancing new treatments that can transform patients’ lives.
Alumis’ lead candidate, ESK-001, is in development for the treatment of moderate-to-severe plaque psoriasis, with pivotal Phase 3 ONWARD trial data expected in the first half of 2026. Additionally, data from the Phase 2b LUMUS trial for systemic lupus erythematosus (SLE) is anticipated in 2026. Meanwhile, ACELYRIN’s development plans for lonigutamab are under evaluation to confirm its differentiation and capital-efficient advancement. Although the specific mechanism of action for lonigutamab has not been disclosed in detail, it is expected to provide a novel approach to treating immune-mediated diseases.
As of December 31, 2024, Alumis and ACELYRIN held approximately $289 million and $448 million, respectively, in cash, cash equivalents, and marketable securities. The combined company is expected to have a pro forma cash balance of approximately $737 million, which is projected to sustain operations through 2027. This funding will support multiple key clinical trial readouts, operating expenses, and capital expenditures. The merger provides financial flexibility, allowing the company to advance its expanded late-stage pipeline, including lonigutamab, while also building commercial capabilities.
This merger represents a powerful strategic and financial combination, enabling the combined company to advance its pipeline across multiple clinical-stage trials while bringing new treatment options to patients. By 2026, with multiple pivotal data readouts, the company expects to demonstrate its product pipeline’s potential and generate significant value for shareholders. Furthermore, the merged company will maintain financial discipline and a flexible capital allocation strategy to maximize the value of its highly differentiated portfolio.
8.Shen Lian Biomedical Ventures into the Human Innovative Drug Sector
On February 6, 2025, Shen Lian Biomedical announced an investment of RMB 60 million in Shizhiyuan Biotechnology, acquiring a 20.48% equity stake in the company. This move marks Shen Lian Biomedical's official entry into the human innovative drug sector, signifying a crucial step toward its transformation into a high-tech biopharmaceutical enterprise. Shizhiyuan Biotechnology is a high-tech company specializing in the research, development, and production of innovative drugs, holding commercialization rights in mainland China for three investigational new drugs targeting allergies, HIV treatment, and herpes simplex virus.
Among them, the HIV treatment monoclonal antibody UB-421 stands out. Originally developed by United Biomedical and licensed from United BioPharma, UB-421 targets the host CD4 receptor, offering a novel therapeutic approach for HIV. Research has demonstrated that UB-421 can significantly increase CD4+ T cell counts and reduce the HIV reservoir without inducing viral resistance mutations. This highlights its significant potential as a direct-acting or adjunctive therapy for patients harboring multidrug-resistant or pan-resistant virus strains. Particularly for patients exhibiting high resistance to most antiretroviral drugs and broadly neutralizing antibodies, UB-421 provides new hope.
Shen Lian Biomedical was the first company globally to apply synthetic peptide technology to veterinary biologics and achieve large-scale commercialization. It has established a comprehensive and leading technological system, particularly excelling in long-chain peptide synthesis. The company has mastered solid-phase synthesis for peptides exceeding 100 amino acids and possesses the capability for stable, kilogram-scale mass production. Additionally, Shen Lian Biomedical is the world's first enterprise to apply circular mRNA technology to veterinary biologics, boasting a complete and independently developed intellectual property system. These technological advantages lay a solid foundation for the company’s expansion into the human innovative drug sector.
Conclusion
Reflecting on the global biopharmaceutical M&A activities in February 2025, several key trends and developments emerge. First, technological innovation remains the core driver of industry growth. Whether it is Pacira BioSciences’ acquisition of GQ Bio to gain access to the high-capacity adenoviral (HCAd) gene therapy vector platform or Gate Neurosciences’ integration of Boost Neuroscience’s expertise to strengthen its leadership in synaptic science research, these investments underscore the importance of cutting-edge technologies in advancing new therapeutic approaches.
Second, with Cosette Pharmaceuticals acquiring Mayne Pharma, we observe how companies leverage mergers to consolidate their market position in specific therapeutic areas and expand geographic reach. This not only enhances drug accessibility but also provides patients with more treatment options.
Additionally, FibroGen’s sale of its China subsidiary to AstraZeneca highlights the potential of cross-border collaborations in optimizing resource allocation, improving financial standing, and advancing R&D projects. Finally, the merger of Alumis and ACELYRIN signals a potential trend where more mid-sized biotechnology firms specializing in specific disease areas may join forces to achieve economies of scale and technological synergy, addressing complex medical challenges collectively.
In summary, the M&A activities in February 2025 reflect dynamic shifts in the biopharmaceutical industry and lay the foundation for future trends. With technological advancements and increasing market globalization, more cross-regional and cross-disciplinary collaborations are expected to emerge, facilitating resource integration and knowledge sharing to propel global healthcare forward. These mergers are not merely financial maneuvers but strategic efforts to explore and shape the future of pharmaceutical innovation, demonstrating the industry’s relentless pursuit of improving public health worldwide.
How to get the latest progress on drug deals?
If you would like to access the latest transaction event information, you can click on the 'Deal' module from the homepage of the Synapse database. Within the Deal module, you can search for global pharmaceutical transaction information using labels such as Drugs, Organization, Target, Drug Type, Deal Date.
Furthermore, you can obtain the original link to the transaction coverage by clicking on the "Deal Name."
In the analysis view, you can see the most active assignors, assignees, popular targets, and other dimensions of analysis, as well as the distribution of research and development statuses at the time of the transaction, to help you better understand the search results.
The Synapse database also supports the ability to view current transactions from the dimension of "drugs" (by selecting "drugs" from the "Adjust Dimension" dropdown menu above). Targeting transactions involving renowned pharmaceutical companies that are of interest to the industry, such as Merck, Roche, etc., Synapse has identified a group of "leading companies" through drugs that have achieved global sales exceeding 1 billion US dollars in 2022. Transactions involving drugs from these leading companies can be filtered by clicking on the "Leading Company" tag on the left-hand side.
In addition to the drug transaction module, you can also view related transaction history on the drug detail page and the institution detail page.
Click on the image below to explore new pharmaceutical funding transactions!
2025-02-08
·药时空
2025年2月6日,申联生物(688098)正式发布对外投资公告,旗下全资子公司上海本天成生物医药有限公司斥资6000万元,将取得创新药企业扬州世之源生物科技有限责任公司20.48%的股权。这一战略举措标志着申联生物正式进军人用创新药领域,在生物科技行业发展的征程中迈出了重要一步。
世之源是一家专注于创新药开发与生产的高科技企业,搭建了先进的单抗垂直整合平台,目前拥有三款在研新药在中国大陆的商业化权益,包括创新抗过敏 Anti-IgE 单克隆抗体药物 (UB-221)、艾滋病治疗单克隆抗体药物 (UB-421) 以及抗单纯疱疹病毒单克隆抗体药物 (UB-621)。三款新药已在多个国家和地区完成了不同阶段的研究,展现出了良好的市场前景和临床价值。其中,艾滋病治疗单克隆抗体药物(UB-421)从台湾联合生物制药股份有限公司(UBP)获得授权并引进中国大陆,该新药由美国联合生物医学公司(UBI)原研研发。2025年1月3日,UB-421的研究成果在自然-医学期刊(Nature Medicine)刊登。这篇原创论文中的受试患者对多数抗逆转录病毒药物、广谱中和抗体及中裕新药的Trogarzo(抗CD4第2结构域抗体)均具有高度的耐药性,并患有卡波西氏肉瘤。研究团队发现,患者T细胞上CD4的第1结构域在整个研究期间皆被UB-421抗体完全占据,HIV病毒无法进入患者宿主细胞进行病毒基因复制,因此在整个长达48周的研究期间都没有观察到病毒耐药突变发生。研究发现,患者表现出对UB-421输注有良好的耐受性,没有发生任何不良事件,CD4+T细胞数也显著增加而HIV储存库减少。这些成果显示了UB-421能以直接作用或辅助药物的形式,用于治疗携带多重耐药或泛耐药病毒的病患,在艾滋治疗史上具有重大意义,可说是一种划时代的新医疗方案。
艾滋病(AIDS)是由人类免疫缺陷病毒(HIV)引起的全球性公共卫生难题。UB-421作为一种创新的抗HIV单克隆抗体药物,通过靶向宿主CD4受体,提供了一种全新的治疗策略。其具备广谱抗病毒活性、长效性和高安全性的特点,为艾滋病治疗领域带来重要突破。未来,UB-421有望在耐药患者管理、简化治疗方案以及功能性治愈研究中发挥重要作用,为HIV感染者带来新的希望。
申联生物作为全球首家将合成肽技术应用于兽用生物制品并实现大规模商业化推广的企业,在生物大分子医药领域已经建立起了一套完整且领先的技术体系。尤其在长链肽技术合成方面处于全球领先地位,已具备长达100个氨基酸以上的超长链的固相合成技术、高度模拟天然结构的空间构象构建技术、公斤级稳定的规模化量产技术等显著优势。公司也是全球兽用生物制品领域首个环状mRNA技术的应用实践企业,已具备全工艺链自主知识产权体系,突破现有技术壁垒。同时,公司积极利用基因工程技术精准开发宠物治疗性单抗,已具备宠物特有靶点的筛选及抗体设计、制备能力。
此次进军人用创新药领域,是申联生物基于自身技术优势做出的重大战略决策,更是申联生物发展历程中又一个重要里程碑。通过本次与世之源的深度合作,申联生物能够将自身前沿的生物技术及生产优势进一步延伸应用,并与世之源的创新药研发能力相结合,实现技术与资源的高效整合,进一步拓展业务版图,提升综合竞争力,强化市场地位。
未来,公司将凭借在前沿生物技术平台和疫苗制备工艺方面的显著优势,在经济动物生物制品领域持续精耕细作,在伴侣动物领域积极拓展版图,在人用生物制品领域不断开拓创新。通过多领域协同发展,逐步成长为世界一流的高科技集团化生物公司,为增进人类健康福祉、提升动物福利贡献更大力量 。
识别微信二维码,可添加药时空小编
请注明:姓名+研究方向!
临床1期
2023-11-09
NEW YORK, Nov. 9, 2023 /PRNewswire/ -- The
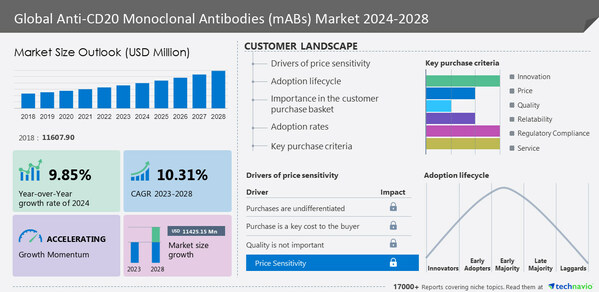
Anti-CD20 monoclonal antibodies (maABs) market is expected to grow by
USD 11.42 billion from 2023 to 2028. In addition, the momentum of the market will progress at a
CAGR of 10.31% during the forecast period, according to Technavio Research. The market has been segmented by product (oncology, neurology, and immunology), type (first generation anti-CD20 monoclonal antibody, second generation anti-CD20 monoclonal antibody, and third generation anti-CD20 monoclonal antibody), and geography (North America, Europe, Asia, and Rest of World (ROW)).
North America is estimated to contribute
57% to the growth of the global market during the forecast period. The growth of the market in North America is due to the high incidence and prevalence of hematological malignancies such as non-Hodgkin lymphoma (NHL).
This report offers an up-to-date analysis of the current market scenario, the latest trends and drivers, and the overall market environment. Read PDF Sample Report
Continue Reading
Technavio has announced its latest market research report titled Global Anti-CD20 Monoclonal Antibodies (mABs) Market 2024-2028
Company Profile:
Acrotech Biopharma Inc., Amgen Inc., AstraZeneca Plc, Celltrion Healthcare Co. Ltd., F. Hoffmann La Roche Ltd., Fosun International Ltd., Genmab AS, IGM Biosciences Inc., JSC BIOCAD, LFB SA, Novartis AG, Pfizer Inc., Regeneron Pharmaceuticals Inc., Spectrum Pharmaceuticals Inc., TG Therapeutics Inc., United BioPharma Inc., and ZHEJIANG HISUN PHARMACEUTICAL Co. Ltd.
Amgen Inc. - The company offers anti-CD20 monoclonal antibodies such as Bax active monomer recombinant antibody 6A7 OAAV00544 and CD52 recombinant antibody campath 1H OAAV00552.
To gain access to more company profiles available with Technavio, buy the report!
Anti-CD20 Monoclonal Antibodies (mABs) Market: Segmentation Analysis
The market share growth by the
oncology segment will be significant during the forecast period. It is the availability of anti-CD20 mAbs in common cancer indications that has driven this segment's growth.
Learn about the contribution of each segment summarized in concise infographics and thorough descriptions. View a PDF Sample Report
"Besides analyzing the current market scenario, our report examines historic data from 2017 to 2021"- Technavio
Anti-CD20 Monoclonal Antibodies (mABs) Market: Market Dynamics
Increased use of combination therapies
High target affinity and specificity of anti-CD20 mABs
Strong pipeline and recent approvals
Key Driver
Increased use of combination therapies is a key factor driving market growth. In cases where the effectiveness and poor tolerability of monotherapy products are worse than in combination therapy, a combination treatment is usually preferable. In combination with other medicines to treat comorbid diseases, the market has seen a strong increase in the use of mABs against CD20.
Major Trend
The development of CD20 bispecific antibodies is the primary trend shaping market growth. Identify key trends, drivers, and challenges in the market. Download a sample to gain access to this information.
Related Reports:
The
breast cancer monoclonal antibodies market size is estimated to grow by USD 15 billion at a CAGR of 12.5% between 2022 and 2027.
The
Biosimilars Market size is estimated to grow by USD 42.23 billion between 2022 and 2027, accelerating at a CAGR of 23% during the forecast period.
What are the key data covered in this anti-CD20 monoclonal antibodies (mABs) market report?
CAGR of the market during the forecast period
Detailed information on factors that will drive the growth of the anti-CD20 monoclonal antibodies (mABs) market between 2023 and 2028.
Precise estimation of the anti-CD20 monoclonal antibodies (mABs) market size and its contribution to the market in focus on the parent market
Accurate predictions about upcoming trends and changes in consumer behavior
Growth of the anti-CD20 monoclonal antibodies (mABs) market across North America, Europe, Asia, and ROW
A thorough analysis of the market's competitive landscape and detailed information about companies
Comprehensive analysis of factors that will challenge the growth of the anti-CD20 monoclonal antibodies (mABs) market companies.
ToC:
Executive Summary
Market Landscape
Market Sizing
Historic Market Sizes
Five Forces Analysis
Market Segmentation by Product
Market Segmentation by Type
Market Segmentation by Geography
Customer Landscape
Geographic Landscape
Drivers, Challenges, & Trends
Company Landscape
Company Analysis
Appendix
About Technavio
Technavio is a leading global technology research and advisory company. Their research and analysis focus on emerging market trends and provide actionable insights to help businesses identify market opportunities and develop effective strategies to optimize their market positions.
With over 500 specialized analysts, Technavio's report library consists of more than 17,000 reports and counting, covering 800 technologies, spanning across 50 countries. Their client base consists of enterprises of all sizes, including more than 100 Fortune 500 companies. This growing client base relies on Technavio's comprehensive coverage, extensive research, and actionable market insights to identify opportunities in existing and potential markets and assess their competitive positions within changing market scenarios.
Contacts
Technavio Research
Jesse Maida
Media & Marketing Executive
US: +1 844 364 1100
UK: +44 203 893 3200
Email: [email protected]
Website:
SOURCE Technavio
100 项与 联合生物制药股份有限公司 相关的药物交易
登录后查看更多信息
100 项与 联合生物制药股份有限公司 相关的转化医学
登录后查看更多信息
组织架构
使用我们的机构树数据加速您的研究。
登录
或

管线布局
2025年09月27日管线快照
管线布局中药物为当前组织机构及其子机构作为药物机构进行统计,早期临床1期并入临床1期,临床1/2期并入临床2期,临床2/3期并入临床3期
药物发现
2
1
临床前
临床1期
1
1
临床3期
其他
9
登录后查看更多信息
当前项目
| 药物(靶点) | 适应症 | 全球最高研发状态 |
|---|---|---|
Semzuvolimab ( CD4 ) | HIV感染 更多 | 临床3期 |
Trastuzumab Biobetter (United BioPharma) ( HER2 ) | HER2阳性胃癌 更多 | 临床1期 |
UB-926 ( HER2 ) | 乳腺癌 更多 | 临床前 |
WO2023061390 ( IgE )专利挖掘 | 消化系统疾病 更多 | 药物发现 |
UB-923 ( CD20 ) | 天疱疮 更多 | 药物发现 |
登录后查看更多信息
药物交易
使用我们的药物交易数据加速您的研究。
登录
或

转化医学
使用我们的转化医学数据加速您的研究。
登录
或

营收
使用 Synapse 探索超过 36 万个组织的财务状况。
登录
或

科研基金(NIH)
访问超过 200 万项资助和基金信息,以提升您的研究之旅。
登录
或

投资
深入了解从初创企业到成熟企业的最新公司投资动态。
登录
或

融资
发掘融资趋势以验证和推进您的投资机会。
登录
或

Eureka LS:
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用
