A Single Arm, Open-labelled Phase II Clinical Trial of Anti-CD19 Chimeric Antigen Receptor Modified T-cell (CAR-T) for Treatment of B-cell Haematological Malignancies
CAR-T therapy is now available as a commercial product for treatment of relapsed /refractory acute lymphoblastic leukaemia and B-lymphoma. There is limited access to this new treatment as the product is very expensive. It is imperative to develop cost effective, closed circuit manufacturing systems for CAR-T cells to make CAR-T cells a point-of care production option. Hong Kong Institute of Biotechnology has established a certified GMP facility and utilize the Prodigy system to manufacture CAR-T cells for clinical application. Prince of Wales Hospital and Hong Kong Children's Hospital will conduct the phase II clinical trial to confirm the efficacy and safety of local manufactured CAR-T cell product.
/ Active, not recruitingN/AIIT Detecting Kidney Transplant Calcineurin Inhibitor Toxicity (the TransTox Study) in adult patients
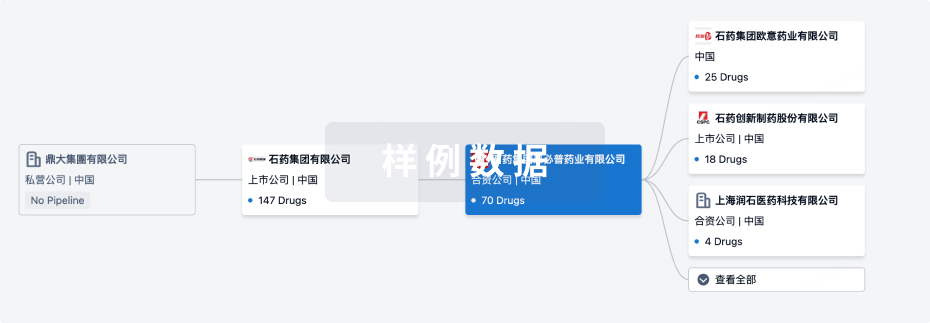
100 项与 Prince of Wales Hospital Foundation Ltd. 相关的临床结果
0 项与 Prince of Wales Hospital Foundation Ltd. 相关的专利(医药)
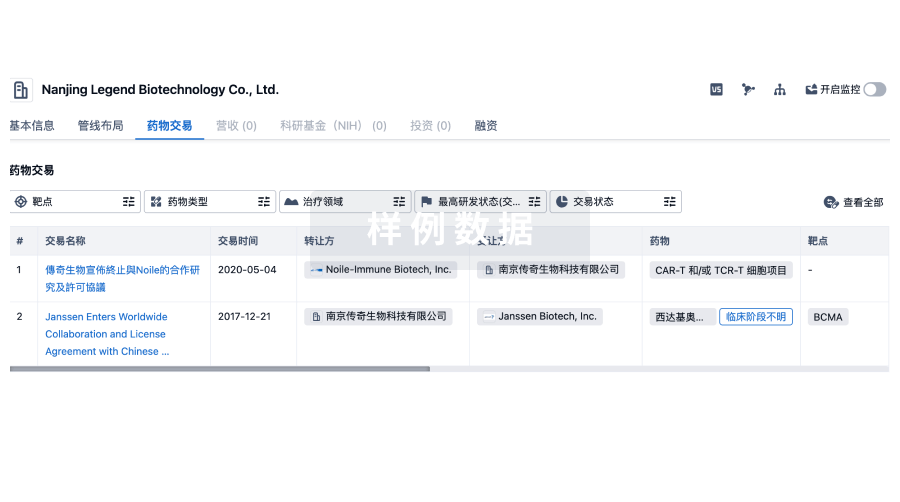
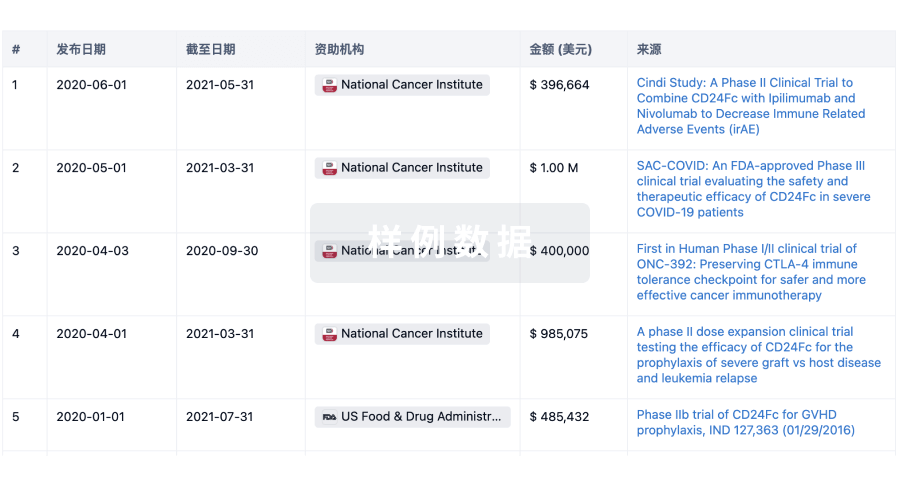
100 项与 Prince of Wales Hospital Foundation Ltd. 相关的药物交易
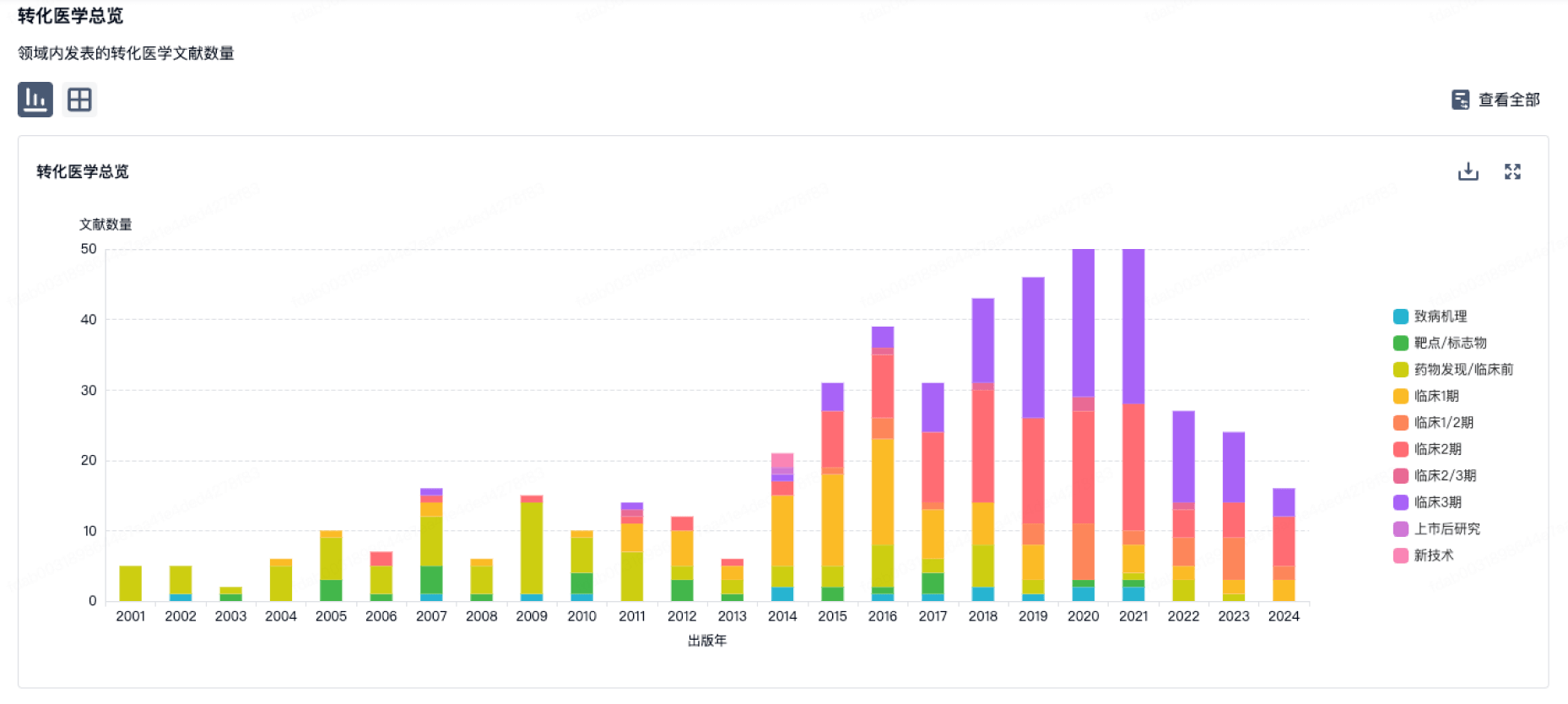
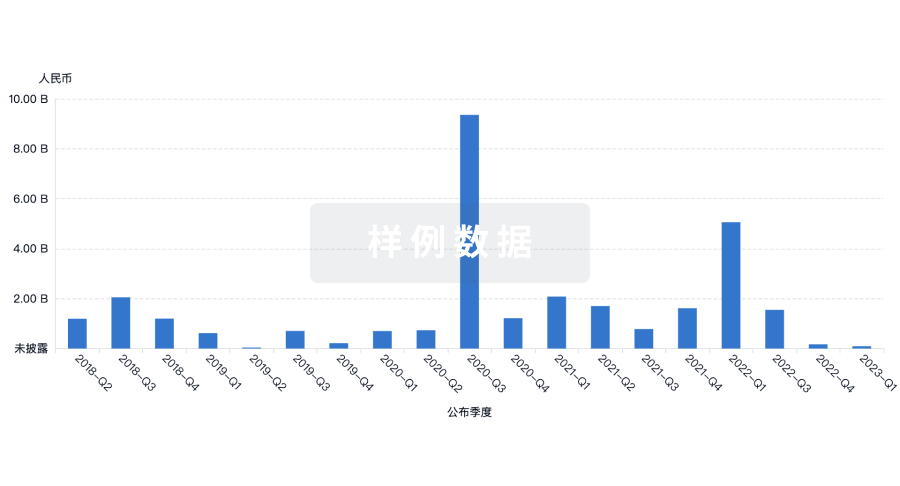
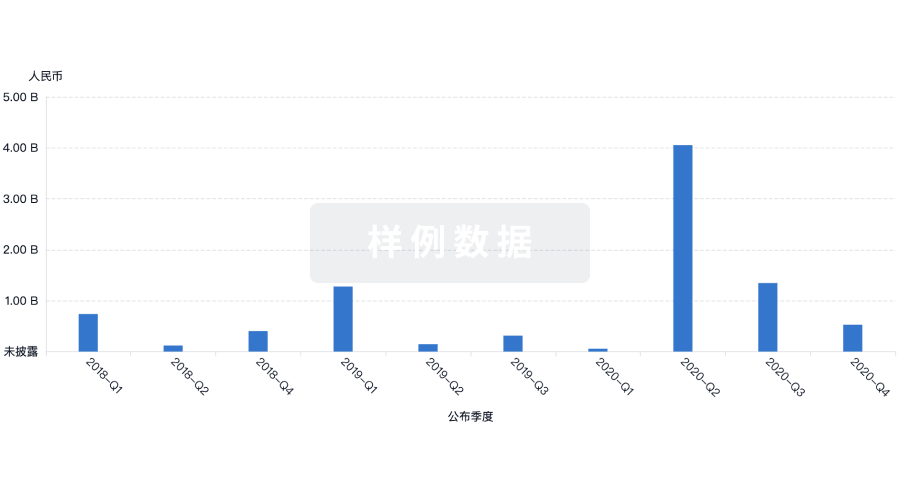
100 项与 Prince of Wales Hospital Foundation Ltd. 相关的转化医学