预约演示
更新于:2025-03-08

Fisabio Foundation
更新于:2025-03-08
概览
关联
2
项与 Fisabio Foundation 相关的临床试验ISRCTN73075133
Microbiome-Genome interaction in two dietary contexts with a gender perspective
开始日期2019-12-30 |
申办/合作机构  Fisabio Foundation Fisabio Foundation [+1] |
EUCTR2019-001186-33-ES
Clinical trial, phase IV, randomized, double-blind, controlled, in children aged 12 to 35 months, of the vaccine against seasonal influenza to estimate efficacy against influenza and other respiratory infections - VIGIRA
开始日期2019-09-27 |
申办/合作机构 |
100 项与 Fisabio Foundation 相关的临床结果
登录后查看更多信息
0 项与 Fisabio Foundation 相关的专利(医药)
登录后查看更多信息
41
项与 Fisabio Foundation 相关的文献(医药)2025-01-01·TRANSPLANTATION PROCEEDINGS
Use of SGLT2i in Non-Diabetic Kidney Transplant Recipients
Article
作者: Calatayud, Emma ; Sancho, Asunción ; Kánter, Julia ; Gandia, Paula ; Quilis, Aina ; Vivó, Elena ; Castro-Alonso, Cristina ; Parra, Manuel ; Gavela, Eva
BACKGROUND:
The potential anti-proteinuric effect of sodium-glucose cotransporter-2 inhibitors (SGLT2i) is of special interest in kidney transplantation. Its benefits have been demonstrated in diabetic kidney transplant recipients (KTRs). We analyzed the efficacy and safety of SGLT2i in non-diabetic KTRs collecting clinical and analytical data at baseline and 6 months after the introduction of the drug.
METHODS:
We performed a unicenter observational study including all non-diabetic KTRs who were prescribed SGLT2i as antiproteinuric therapy. We analyzed clinical and analytical data at baseline and after 6 months of starting SGLT2i.
RESULTS:
We included 22 patients (68.2% men), median age 58 years, with a median post-transplant period of 67 months. Hypertension was present in 95% of the patients, 90% had dyslipidemia, and 68.2% were receiving treatment with renin-angiotensin-aldosterone system blockade agents plus antialdosterone drugs. The most used SGLT2i was dapagliflozin (91%). The median baseline creatinine was 2.1 (1.48 to 2.85) mg/dL, estimated glomerular filtrate rate (eGFR) 31 (23.7 to 45,2) mL/min, and urinary protein/creatinine ratio 1.68 (0.58 to 2.9) g/g. After 6 months of follow-up, we found a significant reduction in proteinuria (1.68 to 1.06 g/g, P = .025), as well as a reduction in uric acid and high-density lipoprotein (HDL). There was a mild significant reduction in eGFR (31 to 28.5 mL/min, P = .001), which was greater in patients with recurrence of the underlying disease or chronic rejection. The drug was discontinued in two patients (9.1%): one due to a urinary tract infection (UTI) and another one due to hemodialysis initiation. There were no cardiovascular events or death.
CONCLUSIONS:
The use of SGLT2i in our cohort was effective to reduce proteinuria in non-diabetic KTRs after 6 months of treatment with an acceptable safety profile.
2024-12-31·Journal of oral microbiology
The effects of nitrate on the oral microbiome: a systematic review investigating prebiotic potential
Review
作者: Rosier, Bob T. ; Burleigh, Mia C. ; Moran, Siobhan P. ; Henriquez, Fiona L.
Background:
Nitrate (NO3-) has been suggested as a prebiotic for oral health. Evidence indicates dietary nitrate and nitrate supplements can increase the proportion of bacterial genera associated with positive oral health whilst reducing bacteria implicated in oral disease(s). In contrast, chlorhexidine-containing mouthwashes, which are commonly used to treat oral infections, promote dysbiosis of the natural microflora and may induce antimicrobial resistance.
Methods:
A systematic review of the literature was undertaken, surrounding the effects of nitrate on the oral microbiota.
Results:
Overall, n = 12 in vivo and in vitro studies found acute and chronic nitrate exposure increased (representatives of) health-associated Neisseria and Rothia (67% and 58% of studies, respectively) whilst reducing periodontal disease-associated Prevotella (33%). Additionally, caries-associated Veillonella and Streptococcus decreased (25% for both genera). Nitrate also altered oral microbiome metabolism, causing an increase in pH levels (n = 5), which is beneficial to limit caries development. Secondary findings highlighted the benefits of nitrate for systemic health (n = 5).
Conclusions:
More clinical trials are required to confirm the impact of nitrate on oral communities. However, these findings support the hypothesis that nitrate could be used as an oral health prebiotic. Future studies should investigate whether chlorhexidine-containing mouthwashes could be replaced or complemented by a nitrate-rich diet or nitrate supplementation.
2024-12-01·BIOCHIMICA ET BIOPHYSICA ACTA-MOLECULAR BASIS OF DISEASE
A disturbed metabolite-GPCR axis is associated with microbial dysbiosis in IBD patients: Potential role of GPR109A in macrophages
Article
作者: Cejudo-Garcés, Andrea ; Seco-Cervera, Marta ; Bauset, Cristina ; Navarro-Vicente, Francisco ; Carda-Diéguez, Miguel ; Calatayud, Sara ; Mira, Álex ; Macias-Ceja, Dulce Carolina ; Cosín-Roger, Jesús ; Buetas, Elena ; Esplugues, Juan Vicente ; Barrachina, María Dolores ; Ortiz-Masiá, Dolores
Inflammatory Bowel Disease (IBD) is a chronic inflammatory disorder of the gastrointestinal tract characterized by disrupted immune function. Indeed, gut microbiota dysbiosis and metabolomic profile alterations, are hallmarks of IBD. In this scenario, metabolite-sensing G-protein coupled receptors (GPCRs), involved in several biological processes, have emerged as pivotal players in the pathophysiology of IBD. The aim of this study was to characterize the axis microbiota-metabolite-GPCR in intestinal surgical resections from IBD patients. Results showed that UC patients had a lower microbiota richness and bacterial load, with a higher proportion of the genus Cellulosimicrobium and a reduced proportion of Escherichia, whereas CD patients showed a decreased abundance of Enterococcus. Furthermore, metabolomic analysis revealed alterations in carboxylic acids, fatty acids, and amino acids in UC and CD samples. These patients also exhibited upregulated expression of most metabolite-sensing GPCRs analysed, which positively correlated with pro-inflammatory and pro-fibrotic markers. The role of GPR109A was studied in depth and increased expression of this receptor was detected in epithelial cells and cells from lamina propria, including CD68+ macrophages, in IBD patients. The treatment with β-hydroxybutyrate increased gene expression of GPR109A, CD86, IL1B and NOS2 in U937-derived macrophages. Besides, when GPR109A was transiently silenced, the mRNA expression and secretion of IL-1β, IL-6 and TNF-α were impaired in M1 macrophages. Finally, the secretome from siGPR109A M1 macrophages reduced the gene and protein expression of COL1A1 and COL3A1 in intestinal fibroblasts. A better understanding of metabolite-sensing GPCRs, such as GPR109A, could establish their potential as therapeutic targets for managing IBD.
100 项与 Fisabio Foundation 相关的药物交易
登录后查看更多信息
100 项与 Fisabio Foundation 相关的转化医学
登录后查看更多信息
组织架构
使用我们的机构树数据加速您的研究。
登录
或
管线布局
2025年04月26日管线快照
无数据报导
登录后保持更新
药物交易
使用我们的药物交易数据加速您的研究。
登录
或
转化医学
使用我们的转化医学数据加速您的研究。
登录
或
营收
使用 Synapse 探索超过 36 万个组织的财务状况。
登录
或
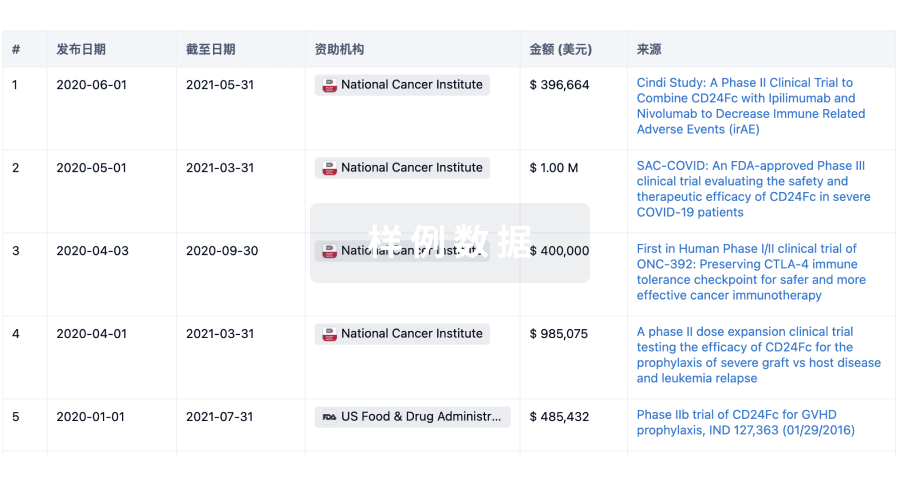
科研基金(NIH)
访问超过 200 万项资助和基金信息,以提升您的研究之旅。
登录
或
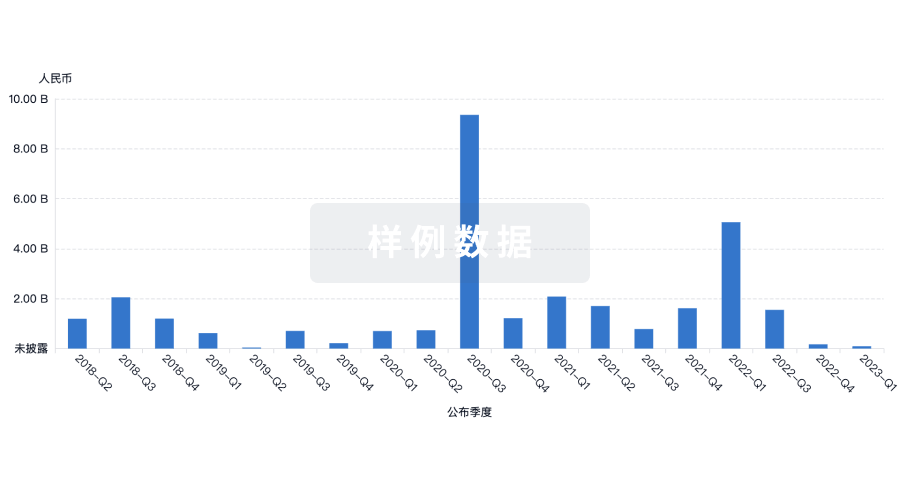
投资
深入了解从初创企业到成熟企业的最新公司投资动态。
登录
或
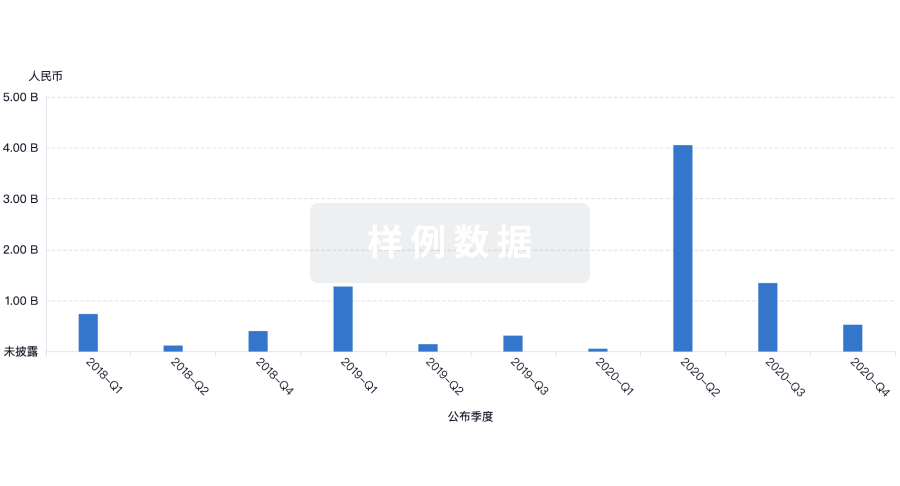
融资
发掘融资趋势以验证和推进您的投资机会。
登录
或
来和Eureka LS聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用