A Single-arm, Open-label Clinical Study to Evaluate the Safety, Tolerability and Preliminary Efficacy of Oncolytic Virus (OVV-01) Injection Combined With or Without Immune Checkpoint Inhibitors in the Treatment of Patients With Advanced Solid Tumors
Phase Ia: To investigate the safety, tolerability and efficacy of OVV-01 injection in the treatment of patients with advanced solid tumors (OVV-01 single dose gradient exploration).
Phase Ib: To evaluate the safety, tolerability and efficacy of OVV-01 injection combined with immune checkpoint inhibitors pembrolizumab (anti-PD-1 monoclonal antibody) or atezolizumab (anti-PD-L1 monoclonal antibody) in the treatment of patients with advanced solid tumors (OVV-01 combined with PD-1/PD-L1 monoclonal antibody dose gradient exploration);
Phase Ic: A cohort expansion of Phase Ib to further analyze the efficacy and safety of OVV-01 injection combined with immune checkpoint inhibitor injection in the treatment of advanced solid tumors.
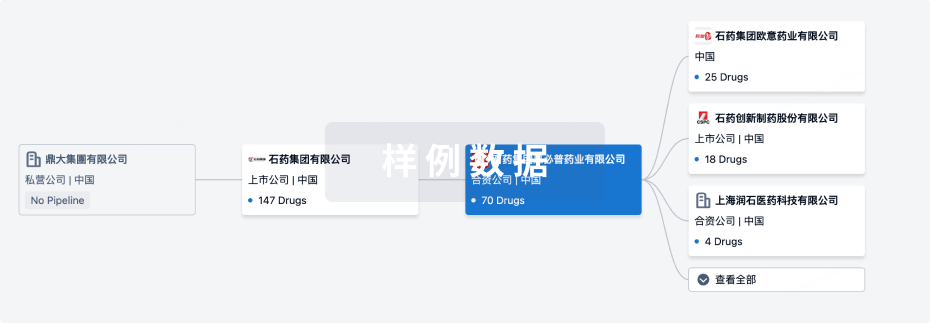
100 项与 Joint Biosciences (HK) Limited 相关的临床结果
0 项与 Joint Biosciences (HK) Limited 相关的专利(医药)
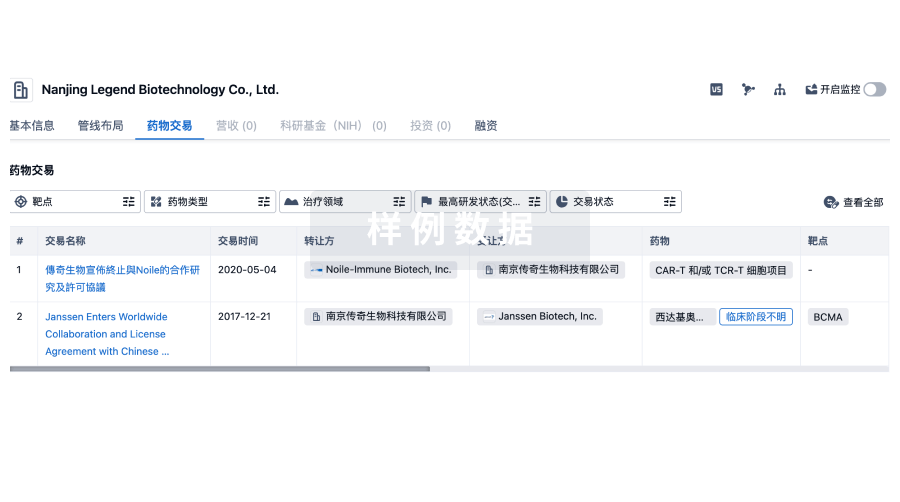
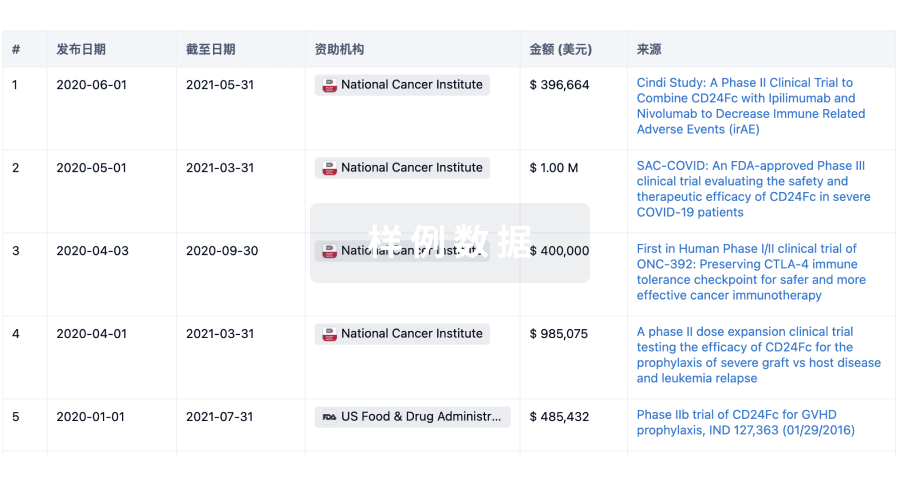
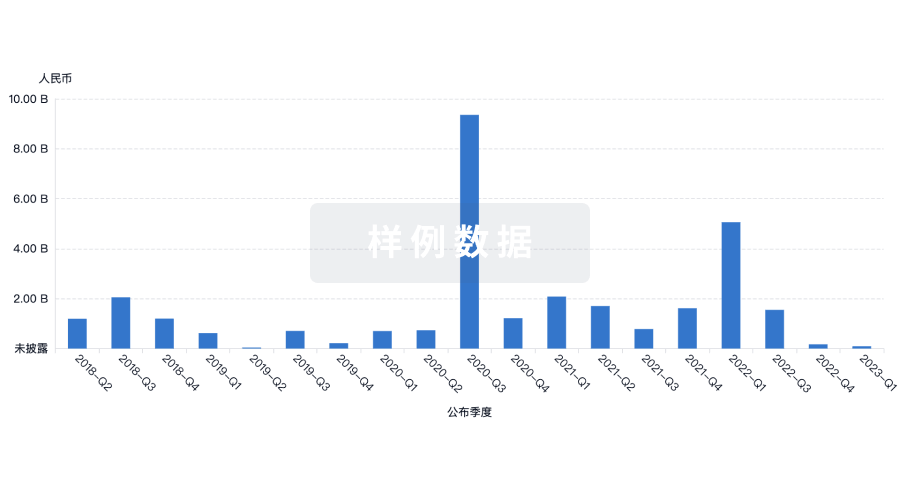
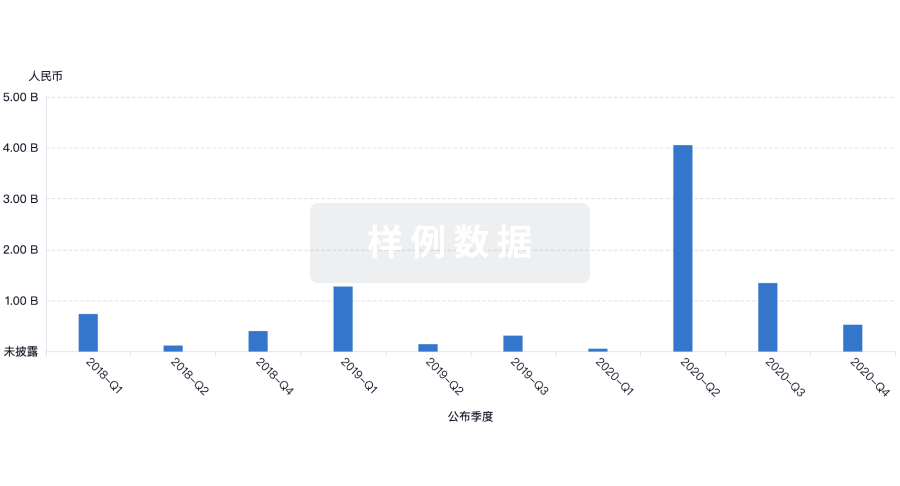
100 项与 Joint Biosciences (HK) Limited 相关的药物交易
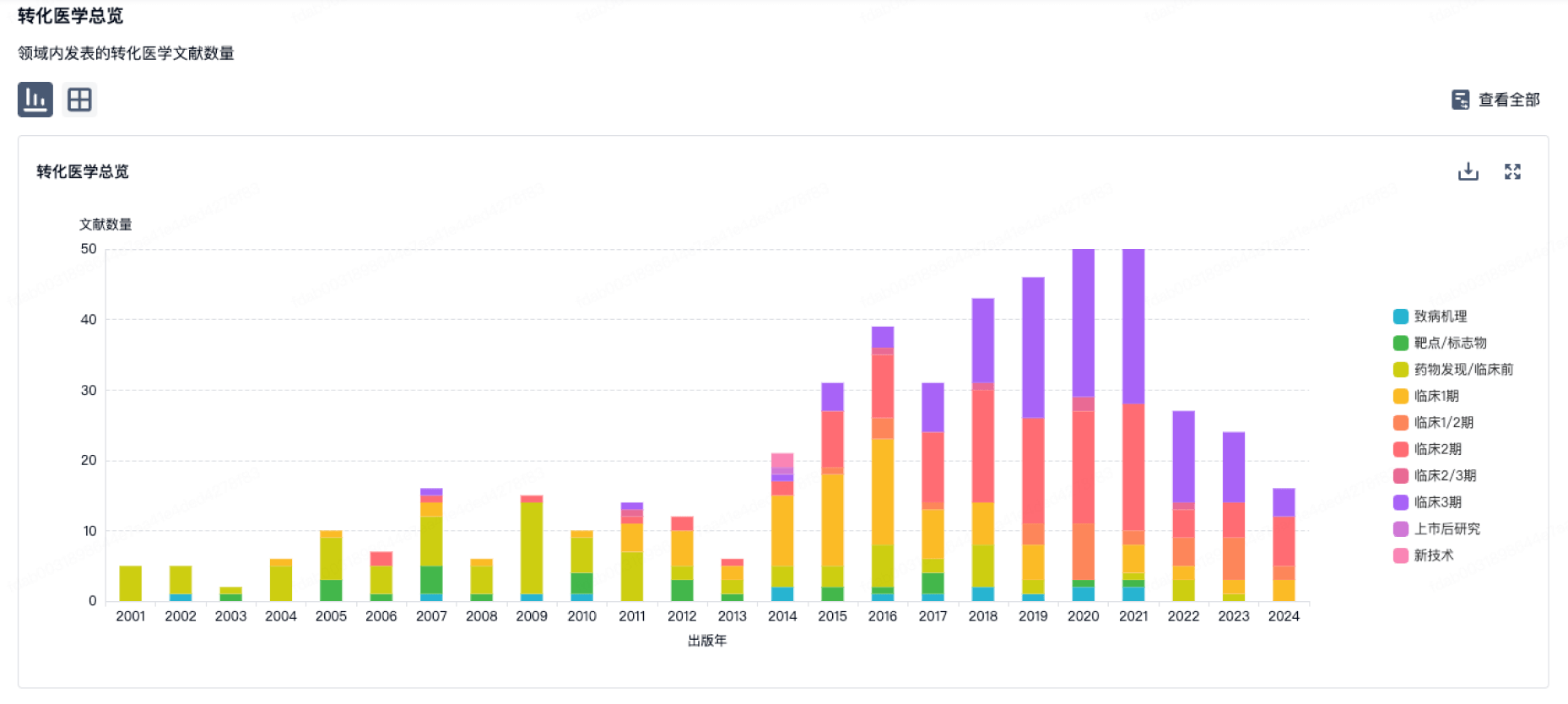
100 项与 Joint Biosciences (HK) Limited 相关的转化医学